Orphan G Protein-Coupled Receptors
Related Symbol Search List
Immunology Background
About Orphan G Protein-Coupled Receptors
Orphan G protein-coupled receptors (OGPRs) are a class of G protein-coupled receptors for which endogenous ligands have not been identified or for which there are no known ligands. They are an important part of the GPCR superfamily and are found in various tissues and cell types throughout the body. Despite their unknown ligands, orphan GPCRs are thought to play important roles in physiological processes and have the potential to be therapeutic targets for various diseases. The study of orphan G protein-coupled receptors aims to gain insight into their potential role in disease development and therapy and to provide possible targets for new drug development. Although molecular biology and bioinformatics techniques have made the identification of orphan GPCRs easy, finding their endogenous ligands has been a challenge. Such studies have given rise to reverse pharmacology approaches that use orphan GPCRs as targets to identify endogenous ligands. This approach has proven to be very successful. The advancement of orphan GPCRs being de-orphanized was even more significant when high-throughput screening techniques were applied to reverse pharmacology. In addition, new neuropeptides were identified.
Table 1 Deorphanized GPCRs reported to be in drug discovery (Chung S, et al., 2008)
| Orphan receptor | Ligand | Therapeutic indication | Action of the compounds |
|---|---|---|---|
| ORL-1 (NOP) | Nociceptin/Orphanin FQ | Stress and pain | Agonist |
| Edg1, 3, 5, 6, 8 | S1P | Autoimmune diseases | Agonist/antagonist |
| H3 | Histamine | Dementia | Antagonist/inverse agonist |
| Orexin1, 2 | OrexinA and B | Sleep disorders | Antagonist |
| SLC-1 (MCH1) | MCH | Obesity, anxiety and depression | Antagonist |
| GHSR | Ghrelin | Catabolic disorders | Agonist |
| GPR38 | Motilin | Gastroparesis and irritable bowl syndrome | Agonist |
| GPRv53 (H4) | Histamine | Inflammation | Antagonist |
| P2Y12 | ADP | Platelet aggregation | Antagonist |
| GPR16 (BLT1) | LTB4 | Inflammation and rheumatoid arthritis | Antagonist |
| BLT2 | LTB4 | Inflammation and rheumatoid arthritis | Antagonist |
| HG55 (CysLT1) | LTD4 | Bronchoconstriction | Antagonist |
| GPR40 | Medium and long fatty acids | Diabetes | Agonist |
| HM74A, B | Nicotinic acid | Dyslipidaemia | Agonist |
Mechanism of Action of Orphan G Protein-Coupled Receptors
The mechanism of action of orphan GPCRs is not fully understood due to the lack of identified ligands. However, several hypotheses have been proposed:
Constitutive Activity: Orphan GPCRs may exhibit basal or constitutive activity in the absence of known ligands. This constitutive activity could regulate intracellular signaling pathways and cellular processes similar to a ligand-activated GPCR.
Endogenous Ligands: Orphan GPCRs may have endogenous ligands that have not been identified or characterized. These ligands can be small molecules, lipids, peptides, or proteins that are present in the cellular microenvironment and play a role in receptor activation.
Ligand-Independent Signaling: Orphan GPCRs may be involved in ligand-independent signaling through interactions with other proteins or receptors. These interactions can activate intracellular signaling cascades and regulate cellular functions without the need for a specific ligand.
The mechanisms of action of orphan G protein-coupled receptors are diverse and complex, and the regulation of their activity is associated with multiple signaling pathways. In the absence of known ligands, these receptors may be regulated through potential endogenous ligands or other protein interactions. Further exploration of their mechanisms of action will help to reveal new signaling pathways and regulatory mechanisms.
Functions of Orphan G Protein-Coupled Receptors
Although the functions of orphan G protein-coupled receptors have not been fully elucidated, it has been shown that they are involved in the regulation of a variety of biological processes, including neurotransmission, immunomodulation, and metabolic regulation. Understanding these receptors can help reveal new biological mechanisms and provide new ideas for disease treatment. Some potential functions include:
Development and Tissue Homeostasis: orphan GPCRs are thought to play a role in embryonic development, tissue differentiation, and maintenance of tissue homeostasis. They may be involved in cell proliferation, migration, and tissue-specific functions during development and regeneration.
Neurological Disorders: Orphan GPCRs have been associated with neurological disorders, including neurodegenerative diseases and psychiatric disorders. Understanding the function and ligands of orphan GPCRs can provide insight into the underlying mechanisms of these diseases and facilitate the development of new therapeutic strategies.
Metabolic Regulation: Orphan GPCRs are thought to be involved in the regulation of metabolic processes such as glucose and lipid metabolism, energy homeostasis, and appetite control. Targeting these receptors may have implications for the treatment of metabolic disorders, including obesity and diabetes.
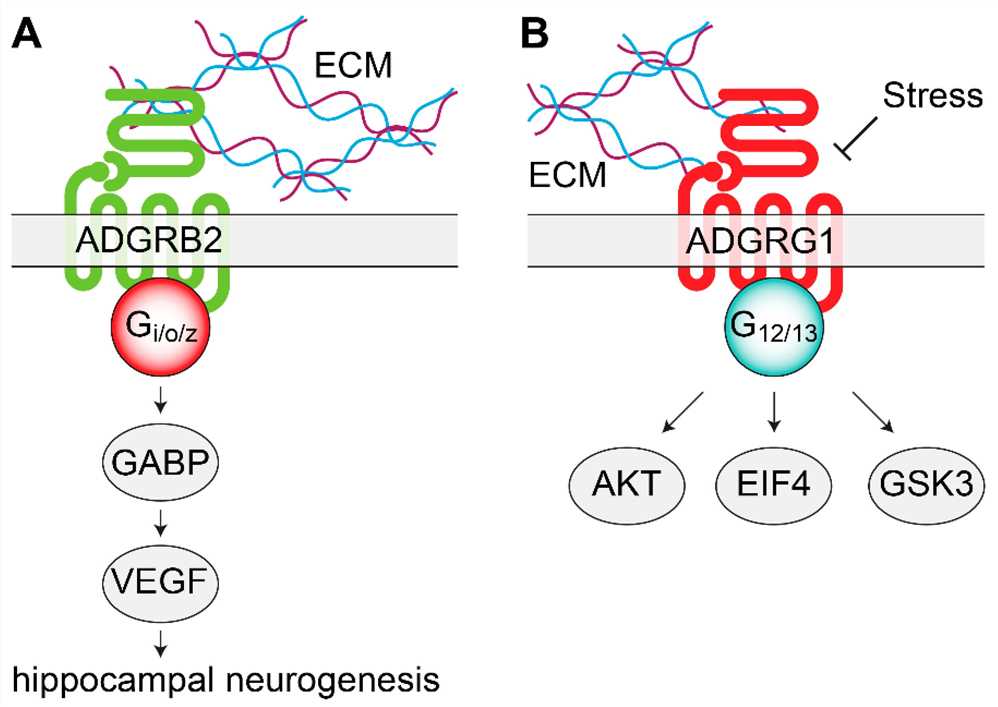 Fig.1 Signaling mechanisms of adhesion orphan G protein coupled receptors (oGPCRs) involved in mood disorders. (Watkins L R, et al., 2020)
Fig.1 Signaling mechanisms of adhesion orphan G protein coupled receptors (oGPCRs) involved in mood disorders. (Watkins L R, et al., 2020)
Available Resources for Orphan G Protein-Coupled Receptors
Orphan G protein-coupled receptors are an interesting group of receptors that have not been fully characterized. Their unknown ligands and mechanisms of action provide exciting opportunities for research and drug discovery. Creative BioMart offers a wide range of products and services related to orphan GPCRs including recombinant proteins, cell and tissue lysates, protein pre-coupled magnetic beads, assay kits, and customized services to support researchers in their studies of these receptors and their potential functions. magnetic beads, assay kits, and customized services to support researchers studying these receptors and their potential functions. These resources provide invaluable tools for studying the mechanism, function, and ligands of orphan GPCRs to reveal their role in a variety of physiological and pathological processes. The following orphan G protein-coupled receptors are displayed, click to view all related molecules/targets and research reagents. Please feel free to contact us with any questions or requests.
References:
- Chung S, Funakoshi T, Civelli O. Orphan GPCR research. Br J Pharmacol. 2008;153 Suppl 1(Suppl 1): S339-S346
- Kim K, Kwon H B, Seong J Y. Cellular and molecular biology of orphan G protein‐coupled receptors[J]. International review of cytology, 2006, 252: 163-218.
- Alavi M S, Shamsizadeh A, Azhdari-Zarmehri H, et al. Orphan G protein-coupled receptors: The role in CNS disorders[J]. Biomedicine & Pharmacotherapy, 2018, 98: 222-232.
- Watkins L R, Orlandi C. Orphan G protein-coupled receptors in affective disorders[J]. Genes, 2020, 11(6): 694.

