Contactins
Related Symbol Search List
Immunology Background
About Contactins
Contactins are a family of cell adhesion molecules that play an important role in the development and function of the nervous system and are involved in cell-cell interactions and signaling processes. Contact proteins consist of several similar proteins encoded by gene families, including CONTACTIN-1, CONTACTIN-2, CONTACTIN-3, CONTACTIN-4, CONTACTIN-5, and others. These proteins are found mainly in vertebrates and are characterized by their modular structure with six immunoglobulin-like domains followed by four fibronectin type III domains.
Contact proteins are expressed in a variety of neural tissues, including the brain, spinal cord, and peripheral nerves. They are mainly localized in cell membranes and mediate cell-cell interactions through homo- or hetero-binding with other cell adhesion molecules.
In recent years, research on contactins has made significant progress. Scientists have revealed the diverse functions of contactins in the nervous system by studying protein structure, expression patterns, and functional properties. Researchers are also using biochemistry, molecular biology, cell biology, and genetics to explore the signaling pathways involved in contactins and their association with neurological diseases.
Contactins play an important role in complex processes that shape the nervous system. Their diverse functions make them an interesting area of research with potential implications for understanding and treating various neurological diseases.
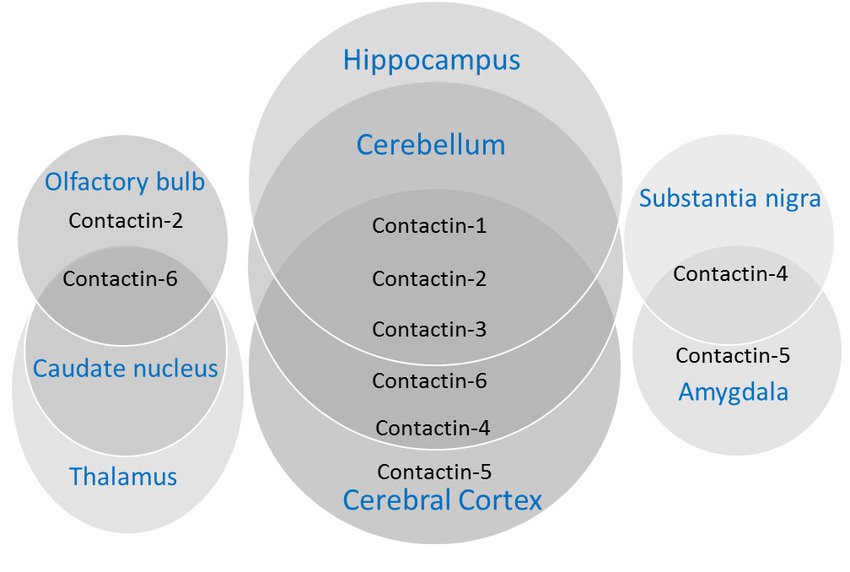 Fig.1 General structure of the contactins. (Chatterjee M, et al., 2019)
Fig.1 General structure of the contactins. (Chatterjee M, et al., 2019)
The six members of the contactin sub-family share a common structure in vertebrates. The extracellular component has immunoglobulin-like domains followed by four fibronectin III-like domains. The protein is attached to the plasma membrane with a glycosylphosphatidylinositol (GPI) anchor and there is no intracellular domain.
Biological Functions of Contactins
Contactins play important roles in various biological processes. Some of the biological functions of contactins include:
Neural Development: Contactins are primarily expressed in the developing nervous system and play a crucial role in neural development. They are involved in processes such as axon outgrowth, guidance, and fasciculation, ensuring proper neuronal connectivity in the developing brain.
Synaptic Function: Contactins are present at the synapses, where they participate in the formation and maintenance of synaptic connections. They facilitate communication between neurons by promoting the clustering and stabilization of synaptic proteins and neurotransmitter receptors.
Myelination: Contactins are also involved in the formation and maintenance of myelin, the fatty substance that surrounds and insulates nerve fibers. They play a role in the adhesion of myelinating cells, oligodendrocytes in the central nervous system, and Schwann cells in the peripheral nervous system, to axons.
Neuronal Plasticity: Contactins have been implicated in mediating neuronal plasticity, which refers to the ability of the nervous system to change and adapt in response to experience and environmental stimuli. They participate in synaptic remodeling, which is crucial for learning, memory, and recovery from injury.
Immune Function: Some contactins, such as Contactin-1 and Contactin-2, are expressed in immune cells and are involved in immune system function. They play a role in regulating immune cell adhesion and migration, as well as modulating immune responses.
Cancer Metastasis: Contactins can also be dysregulated in cancer. Studies have shown that aberrant expression of contactins, particularly Contactin-1, is associated with increased cancer cell invasion and metastasis.
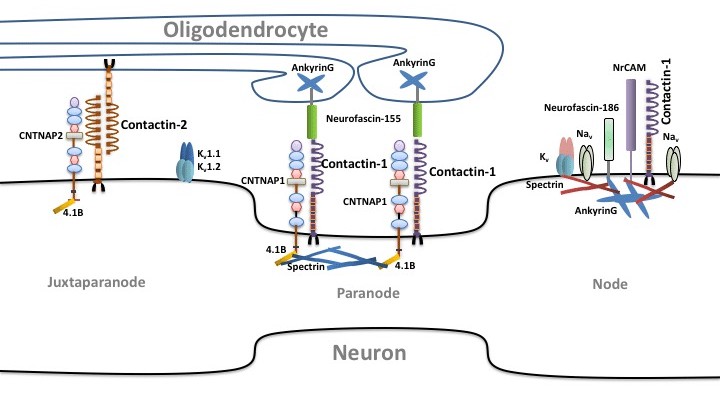 Fig.2 Contactin-1 and contactin-2 in axonal domain organization. (Chatterjee M, et al., 2019)
Fig.2 Contactin-1 and contactin-2 in axonal domain organization. (Chatterjee M, et al., 2019)
CNTNAP: Contactin associated protein; Na V: sodium channel, voltage-gated, type II; K V: Potassium channel, voltage gated; NrCAM: neuronal cell adhesion molecule.
Application of Contactins
Neurodevelopmental Disorders: Mutations in Contactin genes have been associated with various neurodevelopmental disorders such as autism spectrum disorders, intellectual disabilities, and developmental delay. Understanding the role of Contactins in these disorders can help in the development of diagnostic tests and potential therapeutic interventions.
Neuroregeneration: Contactins have shown potential in enhancing nerve regeneration and repair following injury. By studying the mechanisms through which Contactins promote nerve growth and guidance, it may be possible to develop strategies to enhance nerve regeneration in cases of peripheral nerve injuries and spinal cord injuries.
Neurological Diseases: Contactins have been implicated in several neurological diseases such as multiple sclerosis and neuropathies. Studying the role of Contactins in these diseases can help in understanding the underlying mechanisms and potentially developing targeted therapies.
Neuronal Communication: Contactins are involved in mediating cell adhesion and promoting the formation and maintenance of axon connections. By studying contactins, we can gain insights into the mechanisms underlying neuronal communication and synaptic transmission. This knowledge can be useful in understanding brain function and potentially developing therapies for disorders related to synaptic dysfunction such as epilepsy and Alzheimer's disease.
Biomarkers: Contactins have been explored as potential biomarkers for certain neurological conditions. For example, elevated levels of specific Contactins have been found in the cerebrospinal fluid of patients with multiple sclerosis, making them promising biomarkers for disease diagnosis or monitoring treatment response.
It should be noted that the study of contactins is still in progress and the current understanding is still limited. With the continuous development of biotechnology and in-depth research, our understanding of contactins will become more detailed and in-depth.
With the in-depth study of contactins, we can expect a wide range of applications for them in the field of neuroscience. First, a deeper understanding of the structure and function of contactins will help reveal the molecular mechanisms of nervous system development and synapse formation, laying a more solid foundation for neuroscience. Second, the relevance of contactins to neurological diseases makes the study of abnormal functions and mutations of these proteins of great clinical significance, providing new directions for the treatment and intervention of related diseases. Finally, by further investigating the signaling pathways of contactins and their interactions with other molecules, useful information can be provided for the development of new drugs and therapeutic strategies.
Available Resources for Contactins
Creative BioMart provides contactin-related recombinant proteins, cell and tissue lysates, and protein pre-coupled magnetic beads, designed to help you with a series of studies from Western blotting, proteome analysis, protein interaction, etc.
We also offer customizable services including protein expression, purification, and assay development, tailored to your specific contactin-related queries.
In addition, we provide a wide range of technical resources, including application notes, experimental protocols, etc., designed to enhance your understanding and experimental success of contactin research.
The following are contactin-related molecules. Click on the molecule/target to view the research reagents.
Reference:
- Chatterjee M, Schild D, Teunissen C E. Contactins in the central nervous system: role in health and disease[J]. Neural regeneration research, 2019, 14(2): 206.

