What is GJA1 Protein
Also distinguished by the name Connexin43 (Cx43), GJA1 protein is a significant cellular component encoded by the GJA1 gene in humans. Predominantly recognized as a gap junction protein, it was primarily discovered in studies pursuing information on how cells physically and chemically communicate with each other to ensure seamless functioning of multicellular organisms. Back in the 1960s and 1970s, intense research in the field led to the discovery and subsequent understanding of this particular protein.
Relating to its gene locus, the GJA1 gene is geographically situated on the long (q) arm of chromosome 6 at position 22.3, defined as 6q22.3. This locus serves as a reference point, aiding scientists in highlighting the precise genomic address of GJA1 gene.
Delving into its structure, the GJA1 protein forms the building block of a type of intercellular channel known as gap junctions. Each Cx43 is "subunit" of a connexon (gap junction) hemichannel, with six connexin proteins forming a connexon. Positioned between the cell membrane, each pair of these channels align and create a functional pathway for cellular communication.
Function of GJA1 Protein
The GJA1 protein, besides sculpting the assembly of Gap Junctions, plays a regulating role in cell growth and differentiation by permitting passage of various ions and solution molecules (smaller than 1 kilodalton) between neighboring cells. This direct transfer cuts down the response time, offering a significant benefit in situations demanding rapid reaction, for instance, cardiac muscle contraction.
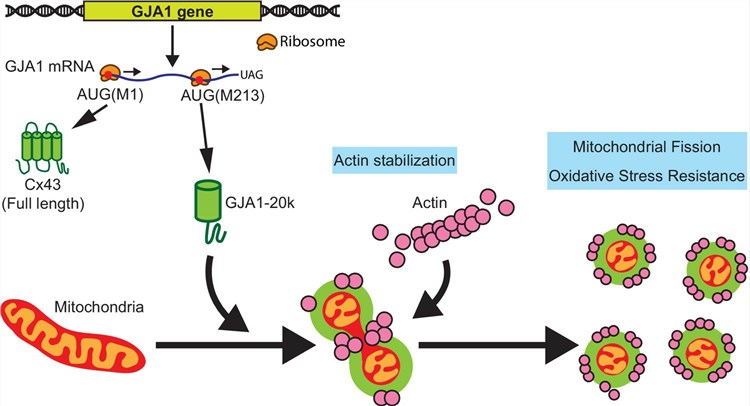
(Daisuke Shimura, et al.2021)
GJA1 Protein-Related Signal Pathway
GJA1 protein-related signal pathways involve the pivotal role in intercellular communication and homeostasis maintenance. This protein majorly contributes to the propagation of calcium wave signaling, essential for excitation-contraction coupling in cardiac cells. There are several signaling pathways concerning GJA1, yet the most common involves kinases such as MAPK/ERK and PKC, which phosphorylate the protein, leading to various effects on channel conductance, assembly, and breakdown.
GJA1 Protein Related Diseases
Alterations in the GJA1 gene can lead to a range of disorders, with the most common being oculodentodigital dysplasia (ODDD), an exceedingly rare condition characterized by alterations in the face, eyes, fingers, and toes. Beyond ODDD, mutations in GJA1 have been associated with other conditions like Hallermann-Streiff Syndrome and syndactyly. Importantly, dysregulated GJA1 expression has been implicated in tumorigenesis, specifically certain types of cancer, where abnormal connexin43 distribution and gap junction communication have been observed.
GJA1 Protein's Applications in Biomedical
Given its crucial role, the GJA1 protein holds distinct potential in various biomedical applications. Examination of GJA1 expression levels can offer distinct clues about cellular health, with either overexpression or underexpression signaling potential problems like cancer.
Furthermore, the manipulation of the GJA1 protein may potentially prove beneficial in treating numerous conditions. For instance, the enhancement of Cx43 expression and gap junction intercellular communication could potentially help in re-establishing controlled cell growth in cancerous cells. Additionally, the protein's role in facilitating intercellular communication makes it a promising target in repairing tissue damage in heart diseases.
In conclusion, the GJA1 protein, or connexin43, is central to various cellular interactions and plays a critical role in maintaining cell homeostasis. Its factoring into several signaling pathways and potential implication in various disorders underlines its importance in disease treatment strategies and biomedical applications. Despite the wealth of knowledge already available about this protein, there remains much about its precisely tuned roles and functions that offer ample scope for further investigation, presenting an exciting avenue of research in the realm of molecular biology and biomedicine.
Our Featured Products
| Cat.No. | Product Name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| GJA1-893M | Recombinant Mouse GJA1 protein, His-tagged | Mouse | E.coli | His |
| Gja1-5414M | Recombinant Mouse Gja1 protein, His-Myc-tagged | Mouse | Yeast | His/Myc |
| Gja1-951M | Recombinant Mouse Gja1 Protein, His-tagged | Mouse | HEK293F | N-His |
| Gja1-1310M | Recombinant Mouse Gja1 protein, His&Myc-tagged | Mouse | HEK293 | His&Myc |
| Gja1-2222M | Recombinant Mouse Gja1 protein, His&Myc-tagged | Mouse | Insect Cell | His&Myc |
| GJA1-3569M | Recombinant Mouse GJA1 Protein, His (Fc)-Avi-tagged | Mouse | HEK293 | His (Fc)-Avi |
Reference
- Daisuke Shimura, Esther Nuebel, Rachel Baum, Steven E Valdez, Shaohua Xiao, Junco S Warren, Joseph A Palatinus, TingTing Hong, Jared Rutter, Robin M Shaw (2021) Protective mitochondrial fission induced by stress-responsive protein GJA1-20k. eLife 10:e69207. https://doi.org/10.7554/eLife.69207

