DGK Family
Related Symbol Search List
Immunology Background
About DGK Family
The DGK (Diacylglycerol Kinase) family is a group of enzymes with EF-hand, C1/Cys-rich zinc finger, and catalytic domains. DGK (Diacylglycerol Kinase) family is a group of enzymes with EF-hand, C1/Cys-rich zinc finger and catalytic domains, which phosphorylate diacylglycerol (DAG) to phosphatidic acid (PA), and mainly regulate the balance between DAG and phosphatidic acid (PA), and play key roles in cell signaling and lipid metabolism.
Studies have shown that diacylglycerol kinases (DGKs) control different life processes in eukaryotes, such as cell differentiation, proliferation, apoptosis, and cytoskeletal reorganization. Currently, 10 DGK isoforms (α-κ) have been identified in mammalian eukaryotic cells, grouped into 5 subfamilies based on the presence of distinct regulatory domains. Among them, DGKα and DGKζ specifically regulate the DAG pool produced as second messengers after stimulation of T cell receptors. Dysregulation of certain DGK isoforms leads to DAG activation in PKC, diminished insulin signaling, and dysregulation of glucose metabolism. DGKs play a role in several physiological events, including cell proliferation and migration, glucose uptake, immunity, and neuronal network construction. DGKs not only regulate antigen-antibody responses in vivo and ex vivo but also modulate tumor cell growth and invasion.
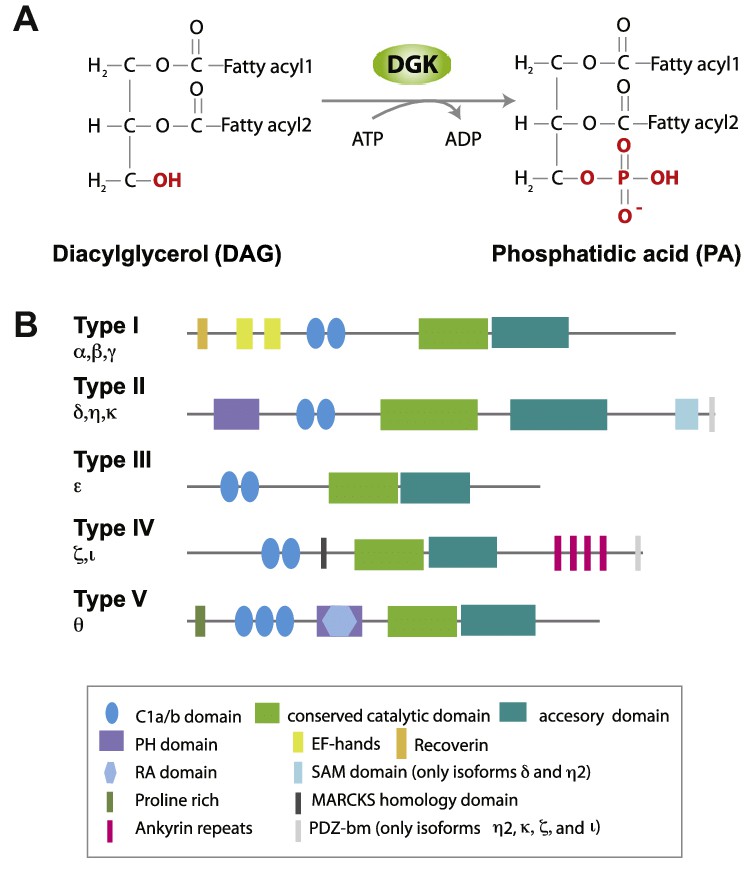 Fig.1 Catalytic function of the DGK family and classification of mammalian DGK. (Rincón E, et al., 2012)
Fig.1 Catalytic function of the DGK family and classification of mammalian DGK. (Rincón E, et al., 2012)
Mechanism of Action of DGKs
Diacylglycerol kinase (DGK) utilizes ATP as a source of phosphate. In non-stimulated cells, DGK activity is low, allowing DAG to be used for glycerophospholipid biosynthesis, but on receptor activation of the phosphoinositide pathway, DGK activity increases, driving the conversion of DAG to PA. As both lipids are thought to function as bioactive lipid signaling molecules with distinct cellular targets, DGK occupies an important position, effectively serving as a switch by terminating the signaling of one lipid while simultaneously activating signaling by another.
Different DGK family members have different substrate specificity and subcellular localization and thus play differentiated functions in different cell types and signaling pathways.
Functions of DGKs
The DGK family plays important functions in cell physiology and pathology.
- DGK family regulates the balance of intracellular DAG and PA, thereby affecting the activation and inhibition of multiple cell signaling pathways, including immune regulation, cell proliferation, apoptosis, and cell migration.
- DGKs are also capable of playing key roles in the pathogenesis of inflammatory responses, autoimmune diseases, and neurodegenerative diseases.
- In addition, DGKs are involved in cytoskeleton remodeling and affect cell morphology and motility.
Therefore, the study of DGKs not only contributes to the understanding of the basic processes of cell biology but also provides new theories and drug development directions for the treatment of related diseases.
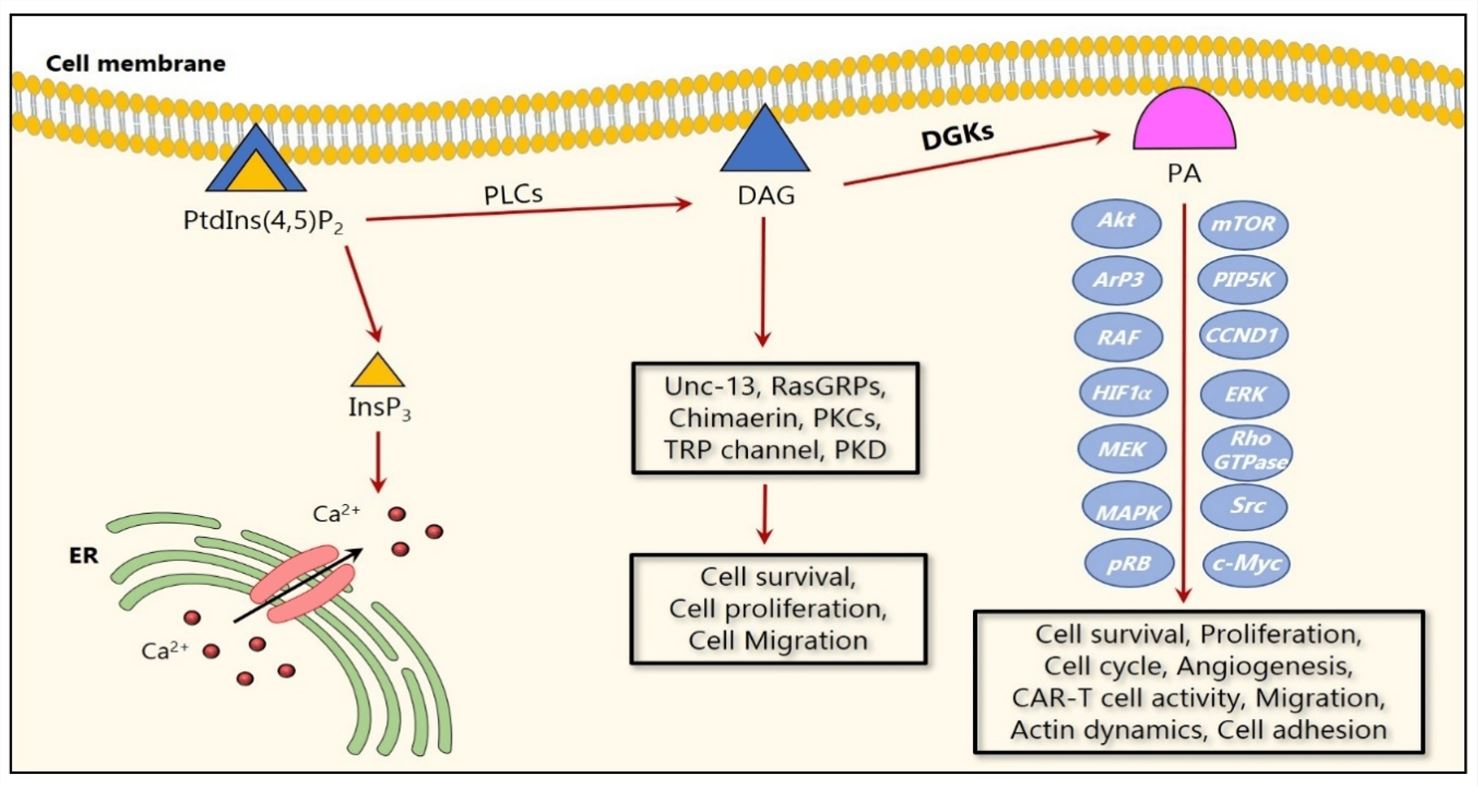 Fig.2 Cartoon representation of DGK signaling in cancer. (Fazio A, 2020)
Fig.2 Cartoon representation of DGK signaling in cancer. (Fazio A, 2020)
Available Resources for DGK Family
Creative BioMart offers a wide range of DGK family-related recombinant proteins, antibodies, enzyme activity assay kits, and other products and services to support your research in the field of the DGK family. The following DGK family are displayed, click to view all related molecules/targets and research reagents. Please feel free to contact us with any questions or requests.
References:
- Rincón E, Gharbi SI, Santos-Mendoza T, Mérida I. Diacylglycerol kinase ζ: at the crossroads of lipid signaling and protein complex organization. Prog Lipid Res. 2012;51(1):1-10.
- Fazio A, Owusu Obeng E, Rusciano I, et al. Subcellular localization relevance and cancer-associated mechanisms of diacylglycerol kinases[J]. International journal of molecular sciences, 2020, 21(15): 5297.

