IVD of Influenza A Virus
🧪 HA-727I
Source: Human Cells
Species: H5N1
Tag: His
Conjugation:
Protein Length: 1-530 a.a.

🧪 HA-731I
Source: Human Cells
Species: H3N2
Tag: His
Conjugation:
Protein Length: 1-531 a.a.

🧪 HA-732I
Source: Human Cells
Species: H5N1
Tag: Fc&His
Conjugation:
Protein Length: 1-527 a.a.

🧪 HA-734I
Source: Human Cells
Species: H5N1
Tag: His
Conjugation:
Protein Length: 1-531 a.a.

🧪 HA-735I
Source: Human Cells
Species: H5N1
Tag: His
Conjugation:
Protein Length: 1-531 a.a.


🧪 HA-736I
Source: Human Cells
Species: H5N1
Tag: His
Conjugation:
Protein Length: 1-531 a.a.

🧪 HA-737I
Source: Human Cells
Species: H5N1
Tag: His
Conjugation:
Protein Length: 1-531 a.a.

🧪 HA-738I
Source: Human Cells
Species: H1N1
Tag: His
Conjugation:
Protein Length: 1-529 a.a.

🧪 NA-308I
Source: Human Cells
Species: H1N1
Tag: Fc
Conjugation:
Protein Length: 36-469 a.a.

🧪 NP-3339I
Source: Insect Cells
Species: H1N1
Tag: His
Conjugation:
Protein Length: 1-490 a.a.

🧪 HA-728I
Source: HEK293
Species: Influenza A Virus
Tag: His
Conjugation:
Protein Length: 1-528 a.a.

🧪 H7N9-01I
Source: Human Cells
Species: H7N9
Tag: His
Conjugation:
Protein Length: 1-524 a.a.

🧪 H7N9-02I
Source: Insect Cells
Species: H7N9
Tag: His
Conjugation:
Protein Length: 1-524 a.a.

Influenza A Virus
Influenza A virus is a common influenza virus that causes influenza in humans and birds. Influenza A viruses, such as H1N1, H5N1, and H7N9, are highly mutable and can easily evade the defenses of the human immune system, posing a serious threat to human health. Symptoms after infection with the virus are mainly high fever, cough, and runny nose, and most patients are accompanied by severe pneumonia. At present, in vitro diagnostic (IVD) methods for influenza use virus culture, antigen detection, and molecular detection.
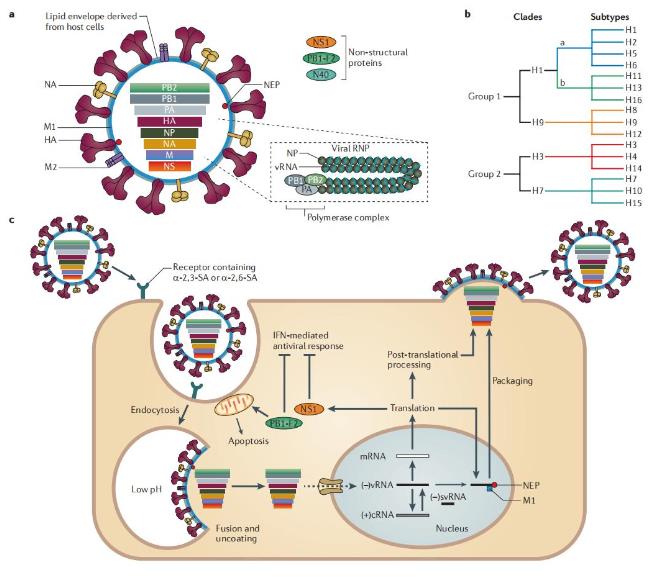 Figure 1. Replication and antigenic classification of influenza A viruses. (Medina R A, et al., 2011)
Figure 1. Replication and antigenic classification of influenza A viruses. (Medina R A, et al., 2011)Main Steps of IVD for Influenza A Virus
- Virus culture. Virus detection by cytopathic effect, hemagglutinin assay, or fluorescent antibody staining.
- Viral antigen testing. The patient's nasopharyngeal secretions were collected for viral antigen detection. If the test result is positive, it indicates that the patient is infected with the influenza A virus.
- Viral nucleic acid detection. Samples such as nasal swabs, throat swabs, nasopharyngeal swabs, tracheal extracts, or sputum from patients are collected for PCR, RT-PCR, and LAMP.
- Microarray chip technology.
Creative BioMart provides high-quality recombinant influenza A protein used for IVD, including ELISA, lateral flow assays, western blots, and other immunoassays.
Highlights of Our Products
- High sensitivity, high specificity, and high purity.
- It is widely used and suitable for downstream immunological experiments.
- Easy to store and transport, conducive to large-scale production and use of vaccines.
- Outstanding success rate and fast development speed.
Our Outstanding Advantages
- IVD proteins can be used to test for a variety of diseases and conditions, making them valuable tools for diagnosing and monitoring health.
- Guarantee high performance, high reliability, and high consistency of protein quality, leading the industry.
- A complete IVD protein platform can provide customized services to meet different scientific research needs.
- High-quality service, high-level experiments, and reliable analysis.
Other Applications
- Virology Studies: Influenza A proteins such as hemagglutinin (HA) and neuraminidase (NA) are extensively studied to understand virus entry, replication, and egress. These studies are crucial for understanding the influenza life cycle and the virus-host interactions.
- Protein Structure and Function: Understanding the structure and function of viral proteins helps in deciphering their role in virus pathogenicity and immune evasion strategies.
- Antigenic Characterization: HA and NA proteins are used to study antigenic drift and shift, which are responsible for seasonal flu variations and potential pandemics.
- Antigen-Based Tests: Proteins like HA, NA, and nucleoprotein (NP) are utilized in assays to detect the presence of influenza virus in clinical samples either through rapid antigen tests or enzyme-linked immunosorbent assays (ELISAs).
- Serological Assays: These assays detect antibodies against influenza A virus proteins in human sera, helping in sero-surveillance and in assessing exposure and immunity within populations.
- Subunit Vaccines: Recombinant HA and NA proteins are used in the production of subunit vaccines to immunize individuals against specific strains of influenza A.
- Virus-Like Particles (VLPs): Influenza virus proteins can be assembled into VLPs, which mimic the virus's structure and are used to create highly immunogenic vaccines without the need for live virus.
- Antiviral Drug Development: Proteins such as neuraminidase are targeted by antiviral drugs, e.g., oseltamivir (Tamiflu). Research into these proteins helps in developing new and improved antiviral agents.
- Monoclonal Antibodies: Monoclonal antibodies targeting influenza A virus proteins can be developed as therapeutic agents to treat or prevent infection.
- Expression Systems: Influenza A virus proteins are commonly used in various expression systems to study their properties, interactions, and to produce proteins for research and commercial purposes.
- Biosensors: Viral proteins like HA are incorporated into biosensors for the rapid detection of influenza A in clinical and environmental samples.
- Immune Response Studies: Influenza A virus proteins are essential in studying host immune responses, both innate and adaptive, which aids in the design of better vaccines and therapies.
Case Study
Case 1: Van Reeth K, Parys A, Gracia JCM, Trus I, Chiers K, Meade P, Liu S, Palese P, Krammer F, Vandoorn E. Sequential vaccinations with divergent H1N1 influenza virus strains induce multi-H1 clade neutralizing antibodies in swine. Nat Commun. 2023 Nov 27;14(1):7745. doi: 10.1038/s41467-023-43339-3. PMID: 38008801; PMCID: PMC10679120.
Vaccines that protect against any H1N1 influenza A virus strain would be advantageous for use in pigs and humans. Here, the authors try to induce a pan-H1N1 antibody response in pigs by sequential vaccination with antigenically divergent H1N1 strains. Adjuvanted whole inactivated vaccines are given intramuscularly in various two- and three-dose regimens.
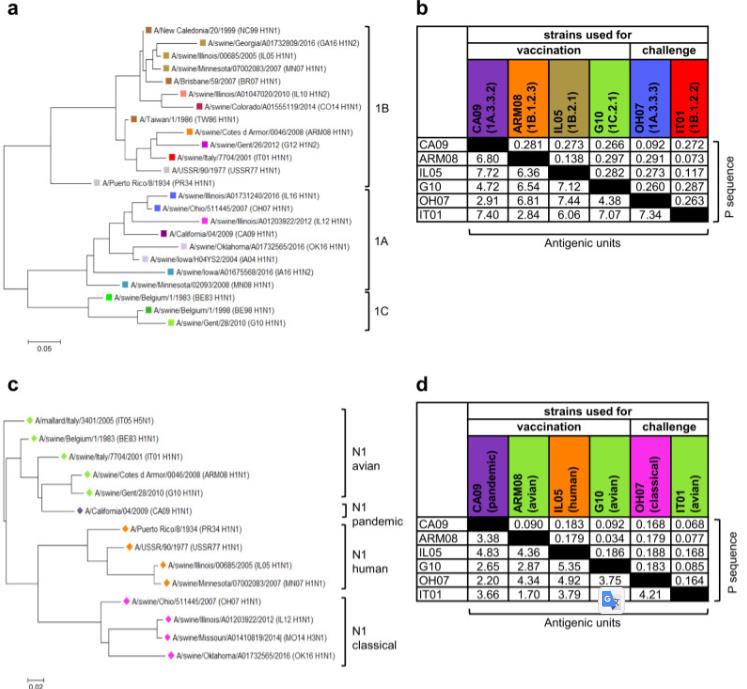 Fig 2. Genetic and antigenic relationships between influenza A virus strains. a, c Phylogenetic trees based on amino acid (aa) sequences of the HA1 (a) and NA (c) of all H1 and N1 strains used. Phylogenetic relationships were estimated using the maximum-likelihood method in MEGA version 7.0 software and the Jones-Taylor-Thornton substitution model. Branch length is proportional to genetic distance. The scale bar indicates aa substitutions per site. Colors were used to indicate H1 lineages (1A, 1B, 1C) and clades, as described by Anderson et al., and N1 lineages (avian, pandemic, human, classical). b, d Genetic and antigenic distances between the HA1 (b) and NA (d) proteins of influenza A virus (IAV) strains used for vaccination and challenge. Genetic distances are expressed as P sequence values (upper right triangle). P sequence is defined as: Number of aa substitutions in the HA1 domain of HA / Total number of aa in the HA1 domain of HA.
Fig 2. Genetic and antigenic relationships between influenza A virus strains. a, c Phylogenetic trees based on amino acid (aa) sequences of the HA1 (a) and NA (c) of all H1 and N1 strains used. Phylogenetic relationships were estimated using the maximum-likelihood method in MEGA version 7.0 software and the Jones-Taylor-Thornton substitution model. Branch length is proportional to genetic distance. The scale bar indicates aa substitutions per site. Colors were used to indicate H1 lineages (1A, 1B, 1C) and clades, as described by Anderson et al., and N1 lineages (avian, pandemic, human, classical). b, d Genetic and antigenic distances between the HA1 (b) and NA (d) proteins of influenza A virus (IAV) strains used for vaccination and challenge. Genetic distances are expressed as P sequence values (upper right triangle). P sequence is defined as: Number of aa substitutions in the HA1 domain of HA / Total number of aa in the HA1 domain of HA.Case 2: Fu X, Wang Q, Ma B, Zhang B, Sun K, Yu X, Ye Z, Zhang M. Advances in Detection Techniques for the H5N1 Avian Influenza Virus. Int J Mol Sci. 2023 Dec 5;24(24):17157. doi: 10.3390/ijms242417157. PMID: 38138987; PMCID: PMC10743243.
Avian influenza is caused by avian influenza virus infection; the H5N1 avian influenza virus is a highly pathogenic subtype, affecting poultry and human health. It was recently found that the H5N1 avian influenza virus tends to spread among mammals. Therefore, early rapid detection methods are highly significant for effectively preventing the spread of H5N1. This paper discusses the detection technologies used in the detection of the H5N1 avian influenza virus, including serological detection technology, immunological detection technology, molecular biology detection technology, genetic detection technology, and biosensors.
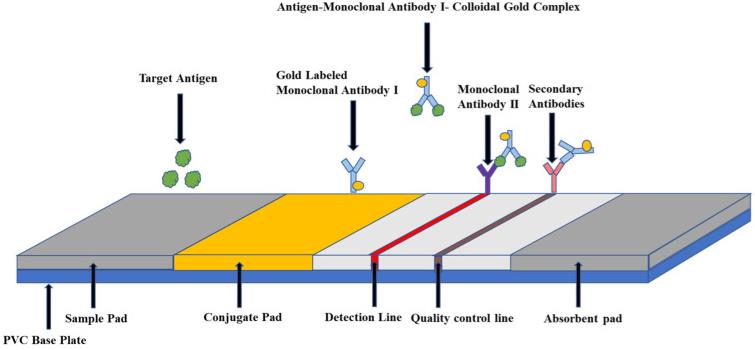 Fig3. Principles of collagen gold immunochromatography technology.
Fig3. Principles of collagen gold immunochromatography technology.Case 3: Burrough ER, Magstadt DR, Petersen B, et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg Infect Dis. 2024 Jul;30(7):1335-1343. doi: 10.3201/eid3007.240508. Epub 2024 Apr 29. PMID: 38683888; PMCID: PMC11210653.
Cow-to-cow transmission appears to have occurred because infections were observed in cattle on Michigan, Idaho, and Ohio farms where avian influenza virus-infected cows were transported. Although the US Food and Drug Administration has indicated the commercial milk supply remains safe, the detection of influenza virus in unpasteurized bovine milk is a concern because of potential cross-species transmission. Continued surveillance of highly pathogenic avian influenza viruses in domestic production animals is needed to prevent cross-species and mammal-to-mammal transmission.
 Fig4. Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary gland tissue sections stained with hematoxylin and eosin. A) Arrowheads indicate segmental loss within open secretory mammary alveoli. Original magnification ×40. B) Arrowheads indicate epithelial degeneration and necrosis lining alveoli with intraluminal sloughing. Asterisk indicates intraluminal neutrophilic inflammation. Original magnification ×400. C, D) Mammary gland tissue sections stained by using avian influenza A immunohistochemistry. C) Brown staining indicates lobular distribution of avian influenza A virus. Original magnification ×40. D) Brown staining indicates strong nuclear and intracytoplasmic immunoreactivity of intact and sloughed epithelial cells within mammary alveoli. Original magnification ×400.
Fig4. Mammary gland lesions in cattle in study of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. A, B) Mammary gland tissue sections stained with hematoxylin and eosin. A) Arrowheads indicate segmental loss within open secretory mammary alveoli. Original magnification ×40. B) Arrowheads indicate epithelial degeneration and necrosis lining alveoli with intraluminal sloughing. Asterisk indicates intraluminal neutrophilic inflammation. Original magnification ×400. C, D) Mammary gland tissue sections stained by using avian influenza A immunohistochemistry. C) Brown staining indicates lobular distribution of avian influenza A virus. Original magnification ×40. D) Brown staining indicates strong nuclear and intracytoplasmic immunoreactivity of intact and sloughed epithelial cells within mammary alveoli. Original magnification ×400.Reference
- Medina R A, García-Sastre A. (2011). Influenza A viruses: new research developments[J]. Nature Reviews Microbiology. 9(8): 590-603.



