What is ARGINASE Protein
Arginase protein is an enzyme that is highly conserved in all kingdoms of life, playing a crucial role in the urea cycle by converting L-arginine to urea and L-ornithine. The process aids in the detoxification of ammonia and the production of nitric oxide, proline, polyamines, and glutamate.
The discovery of arginase dates back to 1904 by two independent researchers, Kossel and Dakin, who discovered the enzyme while studying the breakdown of arginine in mammalian tissues. Over the years, advancements in techniques and technology have led to comprehensive studies and a better understanding of the intricate structure and function of the arginase protein.
The arginase protein originates from the ARG1 and ARG2 genes, located at the human gene locus on chromosome 6q23 and 14q24.1, respectively. ARG1 primarily expresses itself in the liver and is involved in the urea cycle, while ARG2 is found in other tissues such as the kidneys and brain.
The arginase protein is a homo-trimer, with each sub-unit comprising about 322 amino acids. The three identical sub-units form a compact pseudohexamer, which looks like a mushroom. Each monomer has an active site containing a binuclear manganese cluster connected by a bridging hydroxide. The binuclear manganese cluster is essential for the protein's catalytic function.
Function of ARGINASE protein
Arginase plays a variety of vital roles in the body. It catalyzes the hydrolysis of arginine, deriving urea and ornithine. In the liver, this transformation is crucial for the urea cycle, which helps detoxify harmful ammonia. Ornithine, the other product, is a precursor in the biosynthesis of polyamines, proline, and glutamate, important for cell proliferation, collagen production, and as a neurotransmitter, respectively.
ARGINASE protein related signal pathway
Arginase protein serves as an integral component of the immune response and is implicated in several immune-related signal pathways. Its presence in macrophages has revealed its role in immune suppression. Arginase consumption of L-arginine deprives nitric oxide synthase (NOS) of its substrate, reducing the production of nitric oxide, which generally helps destroy invading microorganisms. This arginase-mediated pathway represents an alternative activation state of macrophages (M2), contrasting the classic activation state (M1), which encourages nitric oxide production.
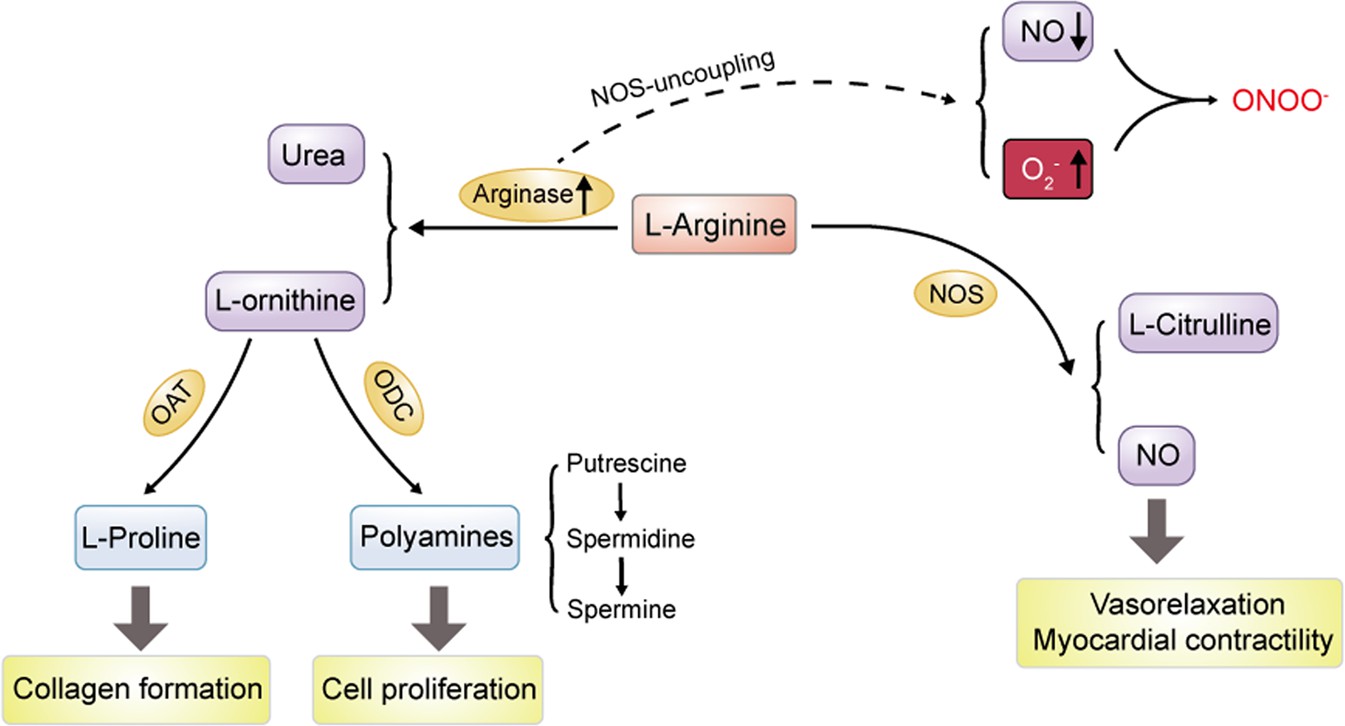
Fig1. Role of arginase metabolism in regulating polyamine and NO production (Li, Z., et al. 2022)
ARGINASE protein related diseases
There's a substantial range of diseases associated with deregulated arginase activity. Arginase deficiency, a rare autosomal recessive disorder, results from a defect in ARG1 and is characterized by hyperargininemia, spastic paraparesis, growth retardation, and mental retardation. More commonly, upregulated arginase activity is seen in asthma, allergic airway inflammation, and various types of cancer. In cancer, this increase supports tumor growth by stifling the immune response and promoting cell proliferation.
ARGINASE protein's applications in biomedical
The biomedical applications of arginase protein have proved invaluable in understanding various disease models and their therapeutic approaches. Given its role in immune regulation, arginase represents a potential therapeutic target in immune-related diseases and cancer. Specific arginase inhibitors are effective in decreasing tumor growth and increasing overall survival in cancer models, with promising progress reported in preclinical studies. In asthma and allergic responses, studies are investigating therapies targeting arginase to prevent airway obstruction and inflammation. Moreover, the use of arginase in diagnostic approaches, by assessing its activity and concentration, serves as a potential biomarker for certain diseases, including liver diseases and certain types of cancer.
In conclusion, arginase is a critical enzyme that plays several crucial roles in human physiological and pathological processes. Although the fundamental knowledge of arginase's structure and function has been known for quite some time, ongoing studies hold promise for improved diagnostic strategies and targeted, successful therapies for a range of diseases. Our understanding of this enzyme continues to evolve as its diverse roles in human health and disease are further unraveled.
Our Featured Products
| Cat.No. | Product Name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| ARG2-108H | Recombinant Human ARG2 protein, T7/His-tagged | Human | E.coli | T7/His |
| ARG2-757H | Recombinant Human ARG2 protein, GST-tagged | Human | Wheat Germ | GST |
| ARG2-372H | Recombinant Human ARG2 Protein, His (Fc)-Avi-tagged | Human | HEK293 | His (Fc)-Avi |
| ARG2-1186HF | Recombinant Full Length Human ARG2 Protein, GST-tagged | Human | In Vitro Cell Free System | GST |
| ARG2-3403H | Recombinant Human ARG2 Protein, Myc/DDK-tagged, C13 and N15-labeled | Human | HEK293T | Myc/DDK |
| ARG2-148HFL | Active Recombinant Full Length Human ARG2 Protein, C-Flag-tagged | Human | Mammalian cells | Flag |
Reference
- Li, Z., Wang, L., Ren, Y., Huang, Y., Liu, W., Lv, Z., Qian, L., Yu, Y., & Xiong, Y. (2022). Arginase: Shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discovery, 8(1), 1-14. https://doi.org/10.1038/s41420-022-01200-4

