Immunology
Creative BioMart Immunology Product List
Immunology Background
Background
Immunology is the branch of biomedical science concerned with the study of the immune system in all organisms. It deals with the physiological functions of the immune system in health and disease; the dysfunctions of the immune system in immunological disorders (autoimmune diseases, hypersensitivity, immunodeficiency, transplant rejection); the physical, chemical, and physiological properties of components of the immune system in vitro, in situ, and in vivo.
History of Immunology
The field of immunology began to take shape in the late 19th and early 20th centuries. One of the key figures in the development of immunology was Edward Jenner, who in 1796 demonstrated that vaccination with cowpox could protect against smallpox, thus laying the foundation for the concept of immunization. Louis Pasteur later expanded on Jenner's work by developing vaccines for rabies and anthrax, further solidifying the importance of immunization.
In the early 20th century, the discovery of antibodies by Emil von Behring and Shibasaburo Kitasato marked a major advance in immunology. They demonstrated that serum from immunized animals could neutralize toxins and confer immunity to other animals, leading to the concept of passive immunity. Understanding of cellular immunity advanced with the work of Elie Metchnikoff, who discovered phagocytosis and emphasized the role of white blood cells in the immune response.
Innate and adaptive immune systems
The immune system can be broadly divided into two main types: innate immunity and adaptive immunity. These two arms of the immune system work together to protect the body from pathogens and other harmful agents.
Innate Immune System
The innate immune system is the body's immediate response to invading pathogens. It includes physical and chemical barriers, such as the skin, mucous membranes, and stomach acid, that prevent pathogens from entering the body. Once a pathogen breaches these barriers, innate immune cells, including macrophages, neutrophils, and natural killer cells, recognize and attack it. These cells have pattern recognition receptors (PRRs) that recognize common molecular patterns on pathogens and trigger an immune response.
Macrophages and neutrophils are phagocytic cells that engulf and destroy pathogens. Dendritic cells act as messengers between the innate and adaptive immune systems, capturing antigens and presenting them to T cells to initiate the adaptive immune response. Natural killer (NK) cells target and destroy infected or abnormal cells.
Adaptive Immune System
The adaptive immune system provides a targeted and highly specific response to pathogens. It is characterized by the clonal expansion of lymphocytes and the generation of immunological memory. The major players in the adaptive immune system are B cells and T cells.
- B Cells and Antibodies: B cells produce antibodies that bind to specific antigens on pathogens. This binding neutralizes the pathogen and marks it for destruction by other immune cells. Memory B cells remain in the body after an infection has been cleared, allowing for a rapid and robust response if the same pathogen is encountered again.
- T Cells: There are several types of T cells, each with a unique role in the immune response. Helper T cells (CD4+ T cells) help other immune cells by secreting cytokines that enhance the immune response. Cytotoxic T cells (CD8+ T cells) directly kill infected or cancerous cells. Regulatory T cells (Tregs) help maintain immune tolerance and prevent autoimmune reactions.
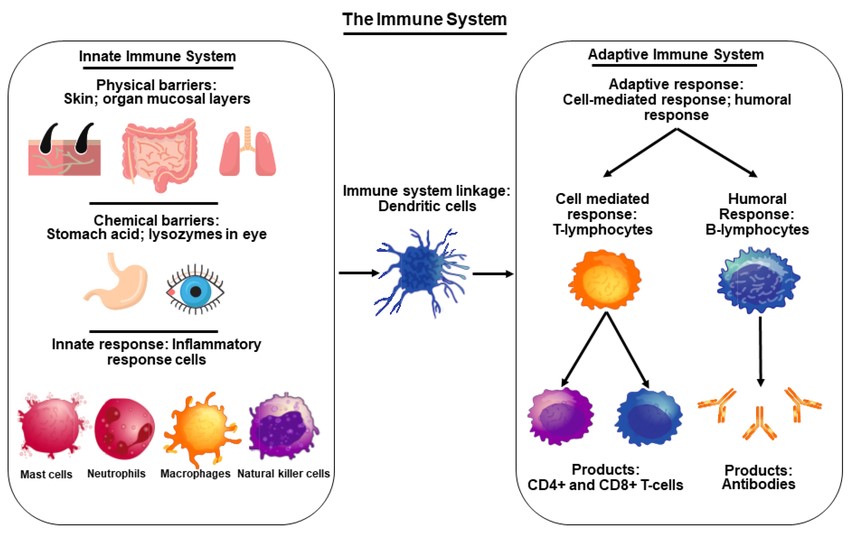 Fig. 1: An overview of the immune system illustrating innate and adaptive immune system components.
Fig. 1: An overview of the immune system illustrating innate and adaptive immune system components.Pathological Aspects
The immune system is directly or indirectly involved in a large number of diseases and other clinically significant processes. These can be differentiated according to the underlying mechanisms.
Defense against pathogens
During infections with bacteria, viruses, protozoa or fungi, the immune system normally defends against the invasion and spread of the pathogens. Under certain conditions, however, the immune response can fail or be inadequate, allowing an infection to spread and not be adequately controlled by the immune system. This can lead to chronic infection, where the pathogens remain in the body and cause permanent or intermittent symptoms. A severe generalized infection, where the infection spreads from a local site of infection through the bloodstream to the entire body, is known as sepsis. This is often fatal due to the massive reactions of the body.
Misdirected or excessive immune response
Autoimmune diseases are caused by a misdirected reaction of the immune system against the body's own structures. These reactions can either lead to the irreversible destruction of the body's own tissues or interfere with the function of the body's own molecules, such as receptors and hormones. Autoimmune diseases include myasthenia gravis, psoriasis, dermatomyositis, type 1 diabetes mellitus, Graves' disease, ulcerative colitis, Crohn's disease, and most inflammatory rheumatic diseases, including rheumatoid arthritis.
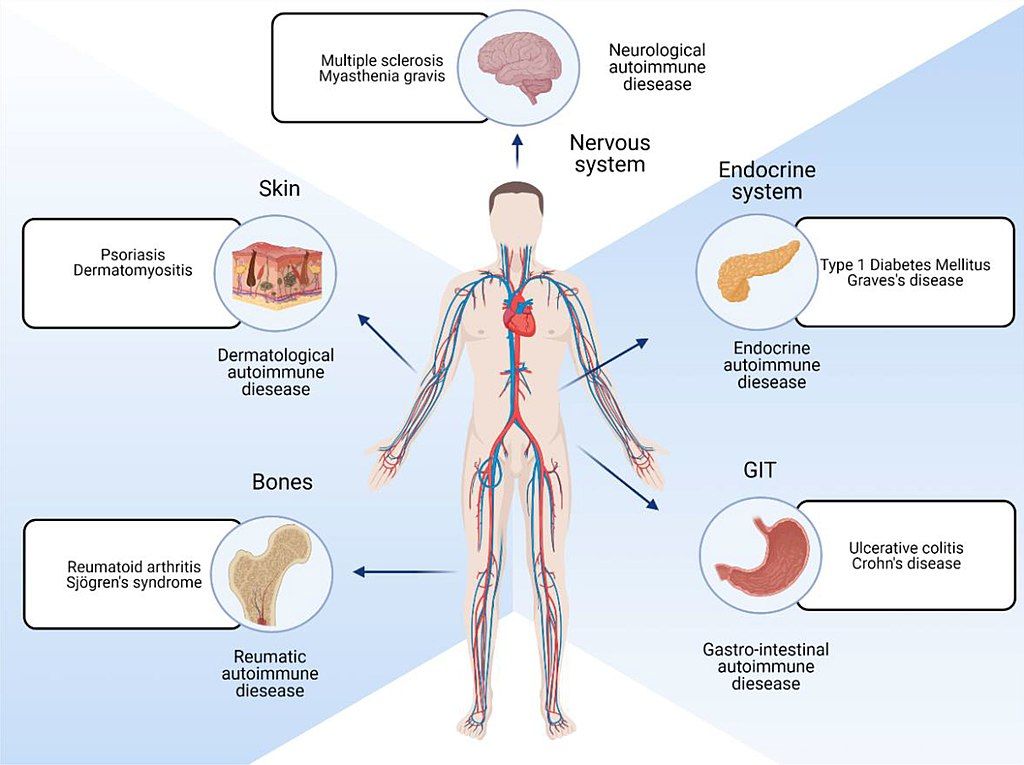 Fig. 2: Parts of body affected by autoimmune diseases.
Fig. 2: Parts of body affected by autoimmune diseases.Allergies, also known as hypersensitivity reactions, are an overreaction of the immune system to certain foreign structures in the body. The development of an allergy requires a harmless initial exposure to the foreign substance, known as the allergen. The body produces antibodies to fight the allergen at the first contact. Any subsequent exposure to the allergen can cause the immune system to overreact. The underlying mechanism is that immunoglobulin E antibodies (IgE) secreted by B cells bind to an allergen and then to a receptor on mast cells or basophils, where they trigger the release of inflammatory chemicals such as histamine and leukotrienes, promote arteriole dilation and vascular permeability, and cause itching, runny nose, mucus secretion, and contraction of pulmonary smooth muscle. Allergies are particularly common to plant pollen, animal dander, food ingredients, and medications. A mixed form of allergy and autoimmune disease is celiac disease, in which there is a cross-reaction to the gluten protein found in most grains and certain structures in the small intestine.
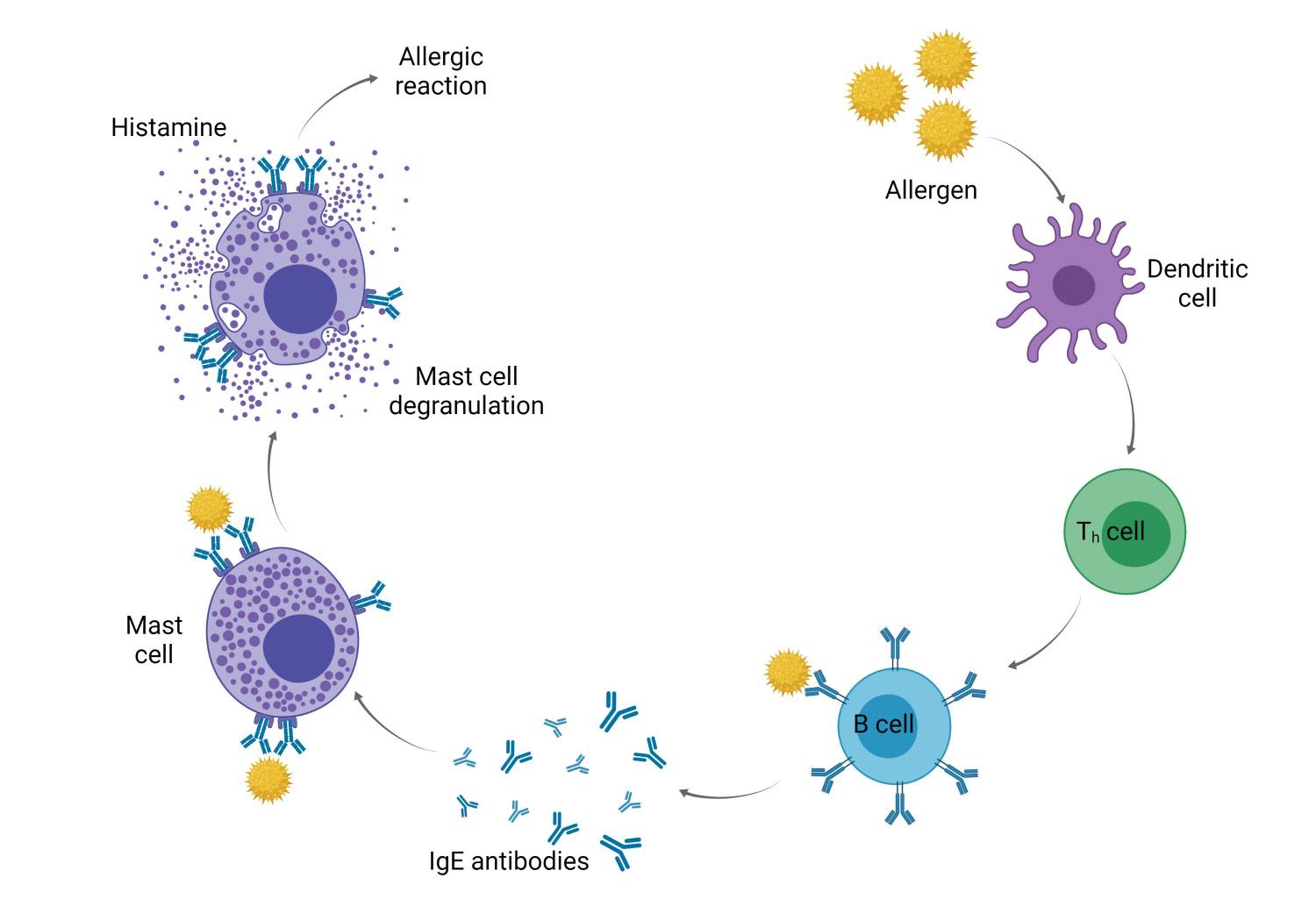 Fig. 3: Mechanism of allergies.
Fig. 3: Mechanism of allergies.Insufficient immune response and immune insufficiency
Diseases characterized by an inadequate immune defense (immune insufficiency) include acquired immunodeficiency syndrome (AIDS), which is caused by infection with the human immunodeficiency virus (HIV). Severe congenital immunodeficiencies in which the humoral and cellular parts of the adaptive immune system are affected simultaneously ("combined") are referred to as severe combined immunodeficiency (SCID). Patients with congenital or acquired immunodeficiency are highly susceptible to infectious diseases, which usually lead to death as the immunodeficiency progresses. Specifically, in the early stages of HIV infection, a rapid burst of viral replication leads to rapid depletion of mucosal CD4+ T cells. This is followed by a slower and progressive depletion of CD4+ T cells in peripheral tissues and blood. HIV has a major impact on gut-associated lymphoid tissue and disrupts intestinal epithelial integrity, resulting in microbial translocation and the onset of chronic immune activation and inflammation. This persistent immune activation further contributes to the progressive depletion of CD4+ T cells, resulting in changes in T cell phenotype and promotion of T cell exhaustion. HIV infection also damages the fibroblastic reticular cell network within lymphoid tissues (LTs), affecting LT architecture and subsequently germinal center responses. HIV is also able to block type I IFN (interferon) induction in human dendritic cells and macrophages. IFN-Is are well-known cytokines that exert broad antiviral effects by inducing the expression of antiviral genes called interferon-stimulated genes (ISGs). The cumulative effect of HIV on the immune system compromises the host's ability to effectively coordinate immune responses against other pathogens or to respond optimally to vaccines. Sufferers develop life-threatening opportunistic infections and tumors.
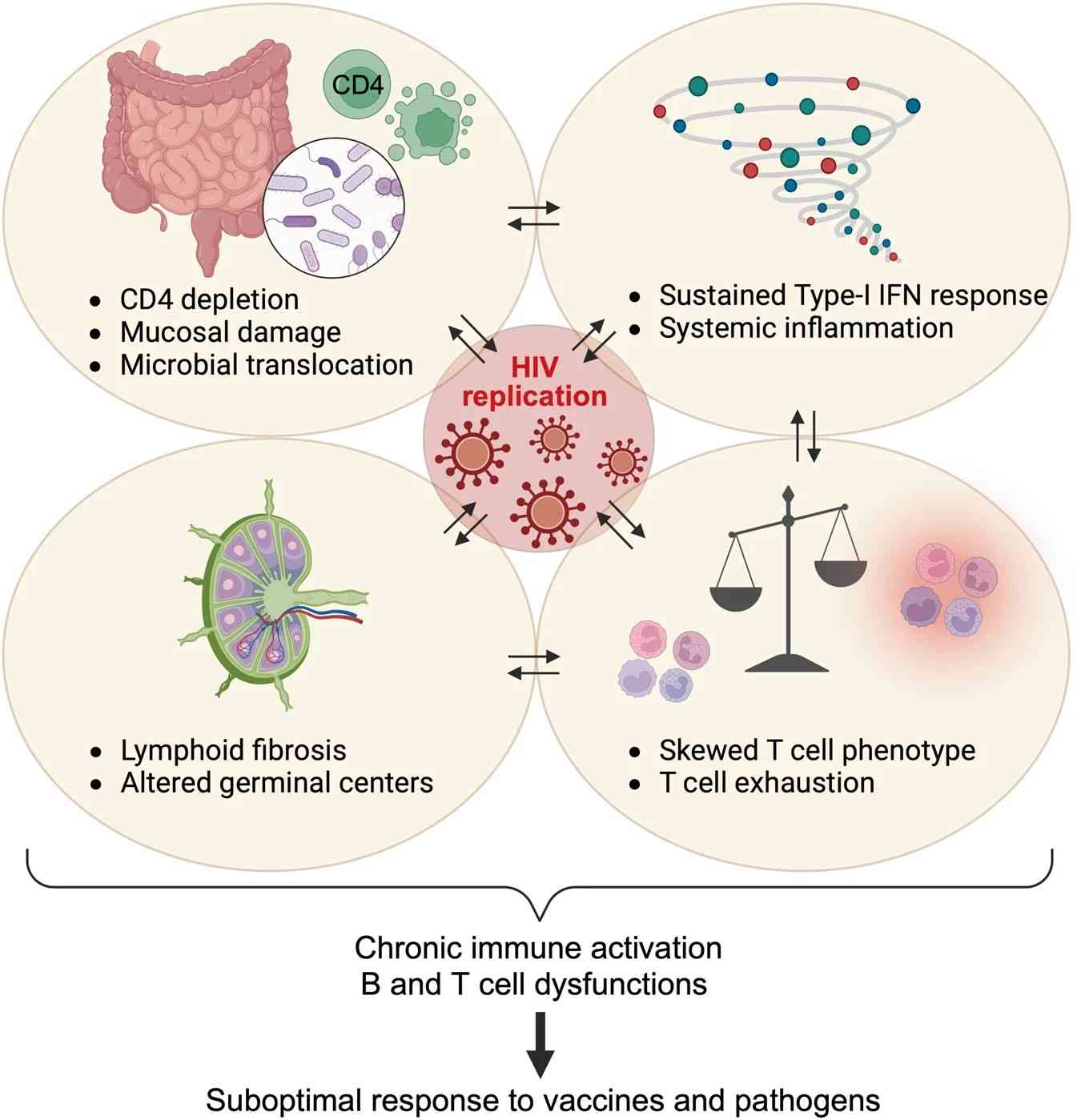 Fig. 4: HIV-associated immune dysregulation.
Fig. 4: HIV-associated immune dysregulation.The immune system also plays an important role in cancer. Patients with immunodeficiency, for example due to immunosuppressive treatment following an organ transplantation or HIV infection, have a significantly higher incidence of certain cancers. The immune system plays a critical role in identifying and eliminating tumors; thus, dysregulation of the immune system can increase the risk of developing cancer. Tumor immunology is the branch of immunology that focuses on the immunological processes involved in tumor initiation, progression and control. Cancer immunotherapy comprises a range of immunological therapeutic approaches.
Immune response against transplants and implants
Immunological processes are of crucial relevance in the transplantation of donor organs. As transplanted organs are recognized by the immune system as foreign to the body, a corresponding immune response occurs. If left untreated, this leads to acute transplantation rejection and thus the loss of function of the organ in question. Conversely, in the case of a stem cell transplant, for example, the immune cells contained in the transplant can also induce an immune response against the recipient organism, known as a graft-versus-host reaction. As a result, to preserve the organ, lifelong treatment of the affected patients with so-called immunosuppressive drugs is necessary, i.e. medication that suppresses the short and long-term immune reactions.
Similar to the transplantation of foreign organs or tissues, the immune system also plays a decisive role in the body's reaction to implants. Implants are made of metals or plastics, for example, and are used for a variety of purposes, including the temporary or permanent replacement of bones or blood vessels, as plastic implants to shape certain body structures and for dental prostheses, as well as to replace or support the function of the body's own organs, such as cochlear implants or pacemakers. As implants are made of exogenous material, they are exposed to a variety of immune defense processes, particularly a chronic inflammatory reaction. The immunological compatibility of these materials is therefore an important aspect of their biocompatibility and makes a decisive contribution to the long-term function of the implant.
Immunotherapy and Advances in Treatment
Advances in immunology have led to the development of various immunotherapies that harness the power of the immune system to treat diseases.
Vaccines stimulate the adaptive immune system to generate memory cells against specific pathogens without causing disease. This principle has been successfully applied to prevent diseases such as measles, polio, and COVID-19.
Monoclonal antibodies are laboratory-made antibodies designed to target specific antigens. They are used to treat cancers, autoimmune diseases, and infectious diseases. For example, monoclonal antibodies that target the HER2 protein are used to treat certain types of breast cancer.
Checkpoint inhibitors block proteins that prevent T cells from attacking cancer cells, thereby enhancing the immune response against tumors. Checkpoint inhibitors have revolutionized the treatment of cancers such as melanoma and lung cancer.
CAR-T cell therapy involves genetically modifying a patient's T cells to express chimeric antigen receptors (CARs) that specifically target cancer cells. CAR T-cell therapy has shown remarkable success in treating certain blood cancers.
Immunomodulators modify the immune response to achieve a desired therapeutic effect. For example, cytokine therapies use interleukins or interferons to boost the immune response against infection or cancer.
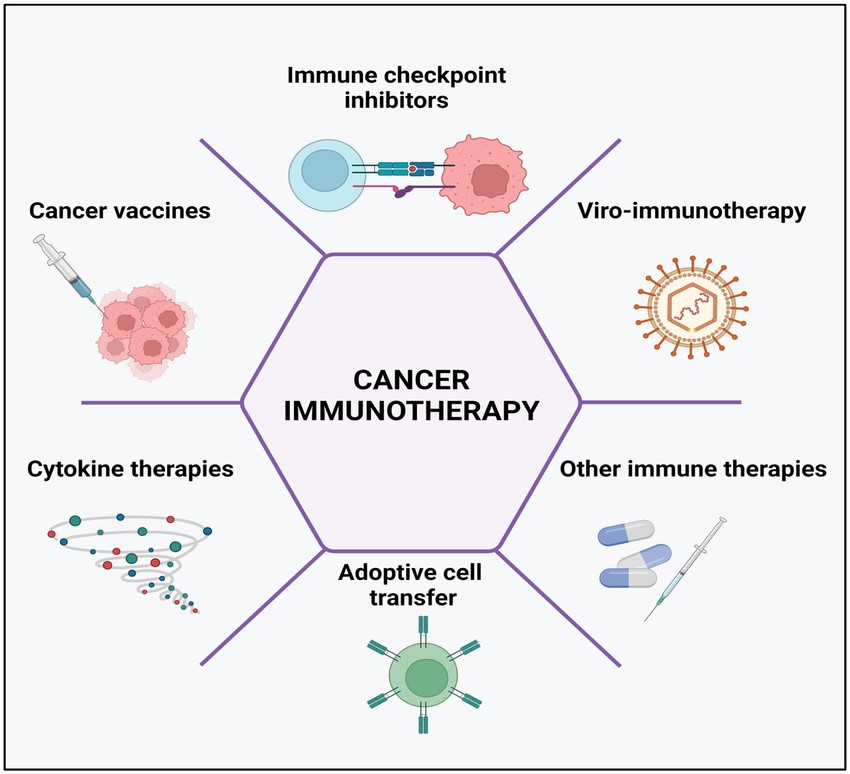 Fig. 5: Cancer immunotherapy types include the use of immune-checkpoint inhibitors, cancer vaccines, cytokines, viruses, and adoptive cell transfer.
Fig. 5: Cancer immunotherapy types include the use of immune-checkpoint inhibitors, cancer vaccines, cytokines, viruses, and adoptive cell transfer.Case Study
Case 1: Pardi, N.; Hogan, MJ.; Weissman, D. Recent advances in mRNA vaccine technology. Current Opinion in Immunology, 2020, 65, 14–20.
Messenger RNA (mRNA) vaccines represent a relatively new vaccine class showing great promise for the future. An mRNA vaccine works by introducing synthetic mRNA encoding the target protein into the body, where it is delivered into cells via lipid nanoparticles; the cells then use this mRNA to produce the target protein, which is displayed on the cell surface, prompting the immune system to recognize it as foreign and mount an immune response, including the production of antibodies and activation of T cells, thereby establishing immunity to the pathogen without using a live virus. This review summarizes the most important developments in mRNA vaccines from the past few years and discusses the challenges and future directions for the field.
One important development is the optimization of mRNA production. Most current mRNA production protocols involve separate enzymatic reactions for DNA template preparation, IVT (in vitro transcription), and 5' capping, with nucleic acid precipitation after each step to remove other reaction components. While current approaches work well at small scale, they are suboptimal for large-scale manufacturing. Two recent innovations have made progress in this direction. The first pertains to a co-transcriptional capping strategy called CleanCap1, which adds a natural 5' cap1 structure to a specific transcription start sequence during IVT. The second methodological innovation has provided an attractive alternative to HPLC purification that is useful at laboratory and industrial scales: Baiersdorfer et al. described a simple method for mRNA purification via adsorption of double-stranded RNA contaminants to cellulose, a cheap and abundant polysaccharide. Taken together, research in the past couple of years has greatly facilitated the large-scale manufacture of therapeutic mRNA, and it is likely that further research in this area will further improve the simplicity and cost-effectiveness of mRNA production.
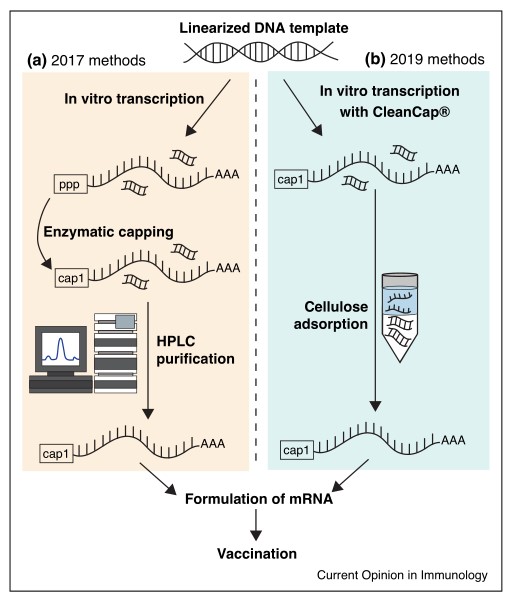 Fig. 6: Schematic of recent innovations in mRNA preparation technology. Shown are (a) methods used by some groups to prepare noninflammatory mRNA circa 2017 (e.g. Pardi et al., 2017) and (b) simplified methods to prepare the same circa 2019.
Fig. 6: Schematic of recent innovations in mRNA preparation technology. Shown are (a) methods used by some groups to prepare noninflammatory mRNA circa 2017 (e.g. Pardi et al., 2017) and (b) simplified methods to prepare the same circa 2019.Case 2: Maude, S. L.; et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. New England Journal of Medicine, vol. 378, no. 5, Feb. 2018, pp. 439–48.
CAR-T therapy, also known as Chimeric Antigen Receptor T-Cell immunotherapy, empowers T cells with new targeted activation functions by introducing an artificially designed CAR molecule into T cells, and then infusing the modified CAR-T cells back into the patient's body, where they are no longer MHC-restricted and can be activated only by binding to the targeted antigen, effectively killing tumor cells. By introducing an engineered CAR molecule into the T cells, the T cells are given a new targeting activation function, and then the modified CAR T-cells are infused back into the patient's body.
In 2017, CTL019 (tisagenlecleucel) from the University of Pennsylvania became the first FDA-approved CAR-T to show impressive results in refractory/recurrent ALL and was approved for the treatment of childhood and young adult ALL in children and adolescents aged 3 to 25 years, opening a new chapter in immunotherapy for malignant hematologic tumors.
In this global study of CAR T-cell therapy, a single infusion of tisagenlecleucel provided durable remission with long-term persistence in pediatric and young adult patients with relapsed or refractory B-cell ALL, with transient high-grade toxic effects. The figure shows overall survival and event-free survival among the 75 patients who received an infusion from the date of tisagenlecleucel infusion to the date of death from any cause. A total of 32 patients had still not had an event at the time of data cutoff. The rate of event-free survival was 73% (95% confidence interval (CI), 60 to 82) at 6 months and 50% (95% CI, 35 to 64) at 12 months. The rate of overall survival among the 75 patients who received tisagenlecleucel was 90% (95% CI, 81 to 95) at 6 months after infusion and 76% (95% CI, 63 to 86) at 12 months after infusion.
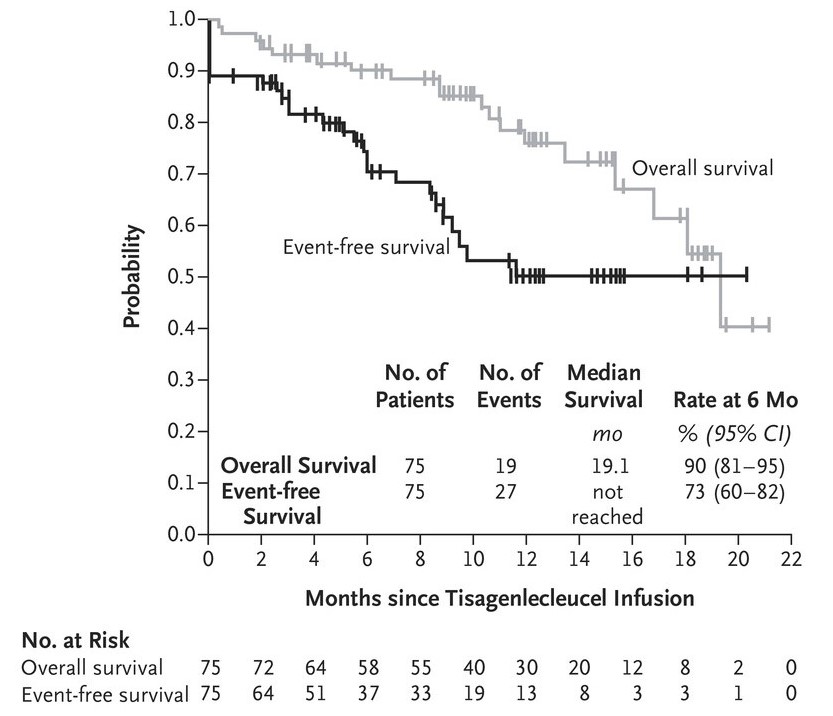 Fig. 7: Event-free and overall survival for 75 patients after tisagenlecleucel treatment.
Fig. 7: Event-free and overall survival for 75 patients after tisagenlecleucel treatment.References
- Höft, Maxine A., et al. "The Immune Response to SARS-CoV-2 in People with HIV." Cellular & Molecular Immunology, vol. 21, no. 2, Feb. 2024, pp. 184–96. www.nature.com, https://doi.org/10.1038/s41423-023-01087-w.
- Kciuk, Mateusz, et al. "Recent Advances in Molecular Mechanisms of Cancer Immunotherapy." Cancers, vol. 15, no. 10, May 2023, p. 2721. DOI.org (Crossref), https://doi.org/10.3390/cancers15102721.
- Manna, Pulak R., et al. "Healthy Immunity on Preventive Medicine for Combating COVID-19." Nutrients, vol. 14, no. 5, Feb. 2022, p. 1004. PubMed Central, https://doi.org/10.3390/nu14051004.
- Maude, Shannon L., et al. "Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia." New England Journal of Medicine, vol. 378, no. 5, Feb. 2018, pp. 439–48. DOI.org (Crossref), https://doi.org/10.1056/NEJMoa1709866.
- Pardi, Norbert, et al. "Recent Advances in mRNA Vaccine Technology." Current Opinion in Immunology, vol. 65, Aug. 2020, pp. 14–20. DOI.org (Crossref), https://doi.org/10.1016/j.coi.2020.01.008.
- Porter, David, et al. "Grading of Cytokine Release Syndrome Associated with the CAR T Cell Therapy Tisagenlecleucel." Journal of Hematology & Oncology, vol. 11, no. 1, Dec. 2018, p. 35. DOI.org (Crossref), https://doi.org/10.1186/s13045-018-0571-y.
- Vairy, Stephanie, et al. "CTL019 (Tisagenlecleucel): CAR-T Therapy for Relapsed and Refractory B-Cell Acute Lymphoblastic Leukemia." Drug Design, Development and Therapy, vol. 12, 2018, pp. 3885–98. PubMed, https://doi.org/10.2147/DDDT.S138765.

