Other Chemokines & Receptors
Related Symbol Search List
Immunology Background
Available Resources for Other Chemokines & Receptors Research
Creative BioMart is your go-to source for all your research needs related to other chemokines and receptors. We offer a carefully curated selection of top-quality products and personalized services to assist you in exploring the intricate world of other chemokines and receptors and their vital role in various physiological processes.
- Our product range includes recombinant proteins, native proteins, GMP proteins, protein pre-coupled magnetic beads, cell and tissue lysates, chromatography reagents, assay kits, and others, all designed to meet your research needs with unrivaled precision and expertise.
- In addition, we provide a wealth of resources on other chemokines and receptors, including in-depth insights on involved pathways, protein functions, interacting proteins, related gene family, and related research area. You can trust Creative BioMart to be your ultimate resource for all things concerning other chemokines and receptors.
Our Featured Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| FAM19A2-7311H | Recombinant Human FAM19A2 protein, hFc-tagged | Human | HEK293 | hFc |
| FAM19A4-279H | Recombinant Human FAM19A4, Fc-tagged | Human | Human Cell | Fc |
| AM19A5-690H | Active Recombinant Human FAM19A5 | Human | E.coli | N/A |
About Other Chemokines & Receptors
Chemokines are a large family of small proteins that play crucial roles in immune cell migration, homeostasis, and inflammation. In addition to C, CC, CX3C, and CXC chemokines and their receptors, there are several other chemokine families and their corresponding receptors that contribute to various physiological and pathological processes, such as atypical chemokine receptor (ACKR1, ACKR2) and FAM19A chemokines.
ACKR (Atypical Chemokine Receptor)
- ACKR1, also known as Duffy Antigen Receptor for Chemokines (DARC), is an atypical chemokine receptor. It is primarily expressed on erythrocytes and endothelial cells. Unlike typical chemokine receptors, ACKR1 does not induce intracellular signaling upon chemokine binding. Instead, it acts as a decoy receptor, binding several chemokines, including CXC, CC, and CX3C chemokines, and preventing their interaction with other chemokine receptors. ACKR1/DARC regulates the availability of chemokines in circulation and modulates chemokine-dependent processes such as leukocyte recruitment during inflammation.
- ACKR2, also known as D6, is another atypical chemokine receptor that functions as a scavenger receptor for CC chemokines. It is primarily expressed on lymphatic endothelial cells and certain subsets of macrophages. ACKR2/D6 selectively internalizes and degrades CC chemokines, thereby controlling their bioavailability. This receptor plays a crucial role in regulating the inflammatory response and immune cell trafficking. It helps to limit excessive inflammation and maintain tissue homeostasis.
FAM19A Chemokines
The FAM19A family of chemokines, also known as TAFA (Tumor Necrosis Factor-Alpha-Induced Protein 8-like Family with Apoptosis-Associated Secretory Phenotype) chemokines, is a relatively newly discovered group of chemokines. They are characterized by the presence of conserved cysteine residues. The FAM19A family includes the following members:
- FAM19A1 (TAFA1)
- FAM19A2 (TAFA2)
- FAM19A3 (TAFA3)
- FAM19A4 (TAFA4)
- FAM19A5 (TAFA5)
The functions and receptor interactions of FAM19A chemokines are still being actively researched. They have been implicated in various physiological processes, including immune regulation, neurodevelopment, and potentially other functions. The specific receptors through which FAM19A chemokines exert their effects are not yet fully characterized, and ongoing studies aim to uncover their roles and interactions with immune cells.
The discovery and characterization of chemokine receptors like ACKR1 and ACKR2, as well as the emerging understanding of FAM19A chemokines, contribute to our knowledge of immune cell migration, inflammation, and the regulation of immune responses. Continued research in these areas may provide insights into novel therapeutic targets and strategies for immune-related disorders and other diseases.
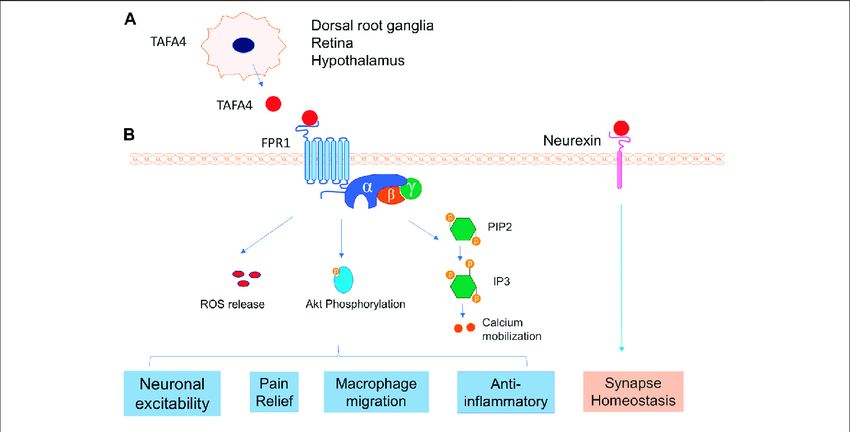 Fig.1 TAFA4 and its receptors interaction. (A) TAFA4is expressed in DRG, retina and hypothalamus. (B) TAFA4 binds FPR1 and activates signalling pathways of Akt phosphorylation, calcium mobilization, and ROS release, leading to various cellular activities. In addition, TAFA4 binds cell surface neurexin and mediates synapse homeostasis. (Zhu S, et al., 2022)
Fig.1 TAFA4 and its receptors interaction. (A) TAFA4is expressed in DRG, retina and hypothalamus. (B) TAFA4 binds FPR1 and activates signalling pathways of Akt phosphorylation, calcium mobilization, and ROS release, leading to various cellular activities. In addition, TAFA4 binds cell surface neurexin and mediates synapse homeostasis. (Zhu S, et al., 2022)
Role of Other Chemokines & Receptors in Immunomodulation and Cell Signaling
The role of FAM19A chemokines and ACKRs in immunomodulation and cell signaling is an area of active research, and our understanding is still evolving. Here are some insights into their potential roles:
FAM19A Chemokines
Research on FAM19A chemokines is relatively recent, and their functions and receptor interactions are still being elucidated. However, studies suggest that FAM19A chemokines may play roles in immunomodulation and cell signaling. Some potential functions include:
- Immunoregulation: FAM19A chemokines might be involved in regulating immune responses. They could influence immune cell activation, migration, and the balance between pro-inflammatory and anti-inflammatory signals.
- Neurodevelopment: FAM19A chemokines have been implicated in neurodevelopmental processes. They may contribute to neuronal migration, axonal guidance, and synaptic connectivity during brain development.
- Inflammatory Diseases: There is emerging evidence that FAM19A chemokines could be associated with inflammatory diseases. Altered expression or dysregulation of FAM19A chemokines might contribute to the pathogenesis of conditions such as autoimmune disorders or chronic inflammation.
However, it's important to note that further research is needed to fully understand the precise roles and mechanisms of FAM19A chemokines in immunomodulation and cell signaling.
Atypical Chemokine Receptors (ACKRs)
ACKRs, including ACKR1 and ACKR2, play unique roles in modulating chemokine signaling and immune responses. Their functions include:
- Scavenging and Decoy Receptor Activity: ACKRs act as scavenger receptors, binding and internalizing chemokines without initiating downstream signaling. This scavenging activity helps regulate the availability and concentration of chemokines in the microenvironment. ACKR1 (DARC) and ACKR2 (D6) perform this function.
- Modulation of Inflammation: ACKR2 (D6) is particularly involved in dampening excessive inflammation. By internalizing and degrading CC chemokines, ACKR2 prevents their interaction with other chemokine receptors, thereby limiting the inflammatory response.
- Immune Cell Recruitment: ACKR1 (DARC) is expressed on erythrocytes and endothelial cells, and its presence can influence immune cell recruitment to specific tissues. By binding and sequestering chemokines, ACKR1 can modulate the migration and trafficking of immune cells.
The precise roles of ACKRs in cell signaling and immunomodulation are still being explored, and their interactions with various chemokines and other signaling molecules are the subject of ongoing research.
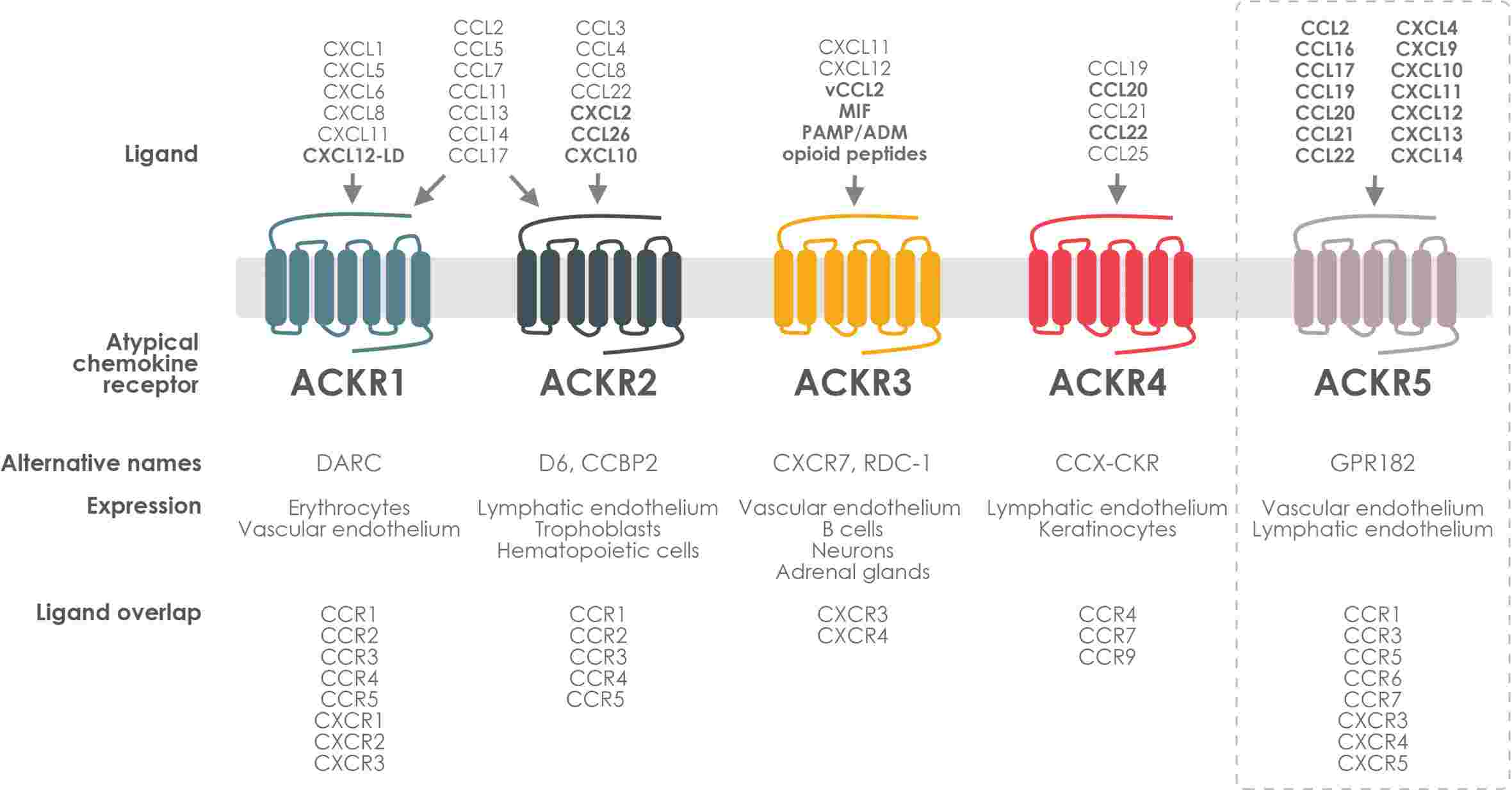 Fig.2 ACKR expression, ligand selectivity and crosstalk with classical chemokine receptors. (Szpakowska M, et al., 2023)
Fig.2 ACKR expression, ligand selectivity and crosstalk with classical chemokine receptors. (Szpakowska M, et al., 2023)
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
Related References
- Zhu S, Hu X, Bennett S, Mai Y, Xu J. Molecular structure, expression and role of TAFA4 and its receptor FPR1 in the spinal cord. Front Cell Dev Biol. 2022;10:911414.
- Wilson GJ, Fukuoka A, Love SR, et al. Chemokine receptors coordinately regulate macrophage dynamics and mammary gland development. Development. 2020;147(12):dev187815.
- Szpakowska M, D'Uonnolo G, Luís R, et al. New pairings and deorphanization among the atypical chemokine receptor family - physiological and clinical relevance. Front Immunol. 2023;14:1133394.

