What is CDC25C Protein
CDC25C, or Cell Division Cycle 25C, is a member of the CDC25 phosphatase family. Also known as Dual Specificity Phosphatase C, it is encoded by the CDC25C gene. This crucial protein plays a pivotal role in cell cycle progression by regulating the transition from G2 phase to mitosis. Synonyms for CDC25C include M-phase inducer phosphatase 3 (MPI-3) and Dual specificity phosphatase C.
Belonging to the larger CDC25 protein family, which includes CDC25A and CDC25B, CDC25C is characterized by its dual-specificity phosphatase activity. Recent research has shed light on its intricate structural characteristics, providing valuable insights into its regulatory functions.
CDC25C Biological Functions and Molecular Mechanisms
The primary biological function of CDC25C is to dephosphorylate and activate the cyclin-dependent kinase 1 (CDK1), also known as Cdc2. This activation is a crucial step in promoting the G2/M transition, allowing the cell to enter the mitotic phase. The activity of CDC25C is tightly regulated to ensure the precise timing of cell division.
At the molecular level, CDC25C acts by removing inhibitory phosphate groups from CDK1, facilitating its activation and subsequent initiation of mitosis. This process is essential for accurate cell division and the maintenance of genomic integrity. Dysregulation of CDC25C can lead to aberrant cell cycle progression and contribute to the development of various diseases.
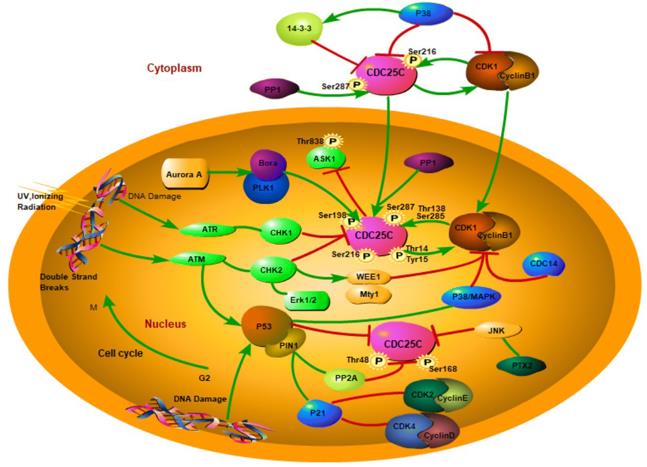
Figure 1. Illustration of CDC25C regulation. (Liu K, et al., 2020)
CDC25C Related Signaling Pathway
CDC25C is intricately involved in the G2/M checkpoint, a critical phase in the cell cycle where the cell ensures that all DNA has been replicated accurately before entering mitosis. The activation of CDC25C is tightly regulated by upstream signaling pathways, including the ATM (ataxia-telangiectasia mutated) and ATR (ataxia-telangiectasia and Rad3-related) pathways.
In response to DNA damage, these pathways activate checkpoint kinases, which phosphorylate and inhibit CDC25C. This inhibition prevents premature entry into mitosis, allowing the cell time to repair damaged DNA. Understanding the signaling pathways that regulate CDC25C provides valuable insights into how cells maintain genomic stability.
CDC25C Related Diseases
The dysregulation of CDC25C has been implicated in several diseases, particularly cancer. Overexpression of CDC25C has been observed in various cancer types, including breast, ovarian, and lung cancers. Elevated levels of CDC25C contribute to uncontrolled cell division and tumor growth, making it an attractive target for cancer therapy.
Additionally, studies have linked CDC25C to neurodegenerative disorders and autoimmune diseases. The intricate balance maintained by CDC25C in cell cycle regulation underscores its significance in health and disease.
CDC25C's Applications in Biomedicine
The unique role of CDC25C in cell cycle regulation has paved the way for its applications in biomedical research. Its involvement in disease, particularly cancer, has made CDC25C an attractive target for therapeutic intervention.
In diagnostics development, CDC25C can serve as a biomarker for certain cancers. Elevated levels of CDC25C in patient samples may indicate abnormal cell cycle progression and could aid in early cancer detection. This biomarker potential extends to prognosis, helping clinicians assess the aggressiveness of tumors and tailor treatment strategies accordingly.
Vaccine development is another area where CDC25C holds promise. By understanding the protein's role in cell cycle regulation, researchers can explore its potential as a target for cancer vaccines. Stimulating an immune response against CDC25C could selectively target cancer cells, providing a novel approach to cancer immunotherapy.
Therapeutically, efforts are underway to develop small molecule inhibitors targeting CDC25C. These inhibitors aim to disrupt the aberrant cell cycle progression observed in cancer cells, potentially offering a new avenue for cancer treatment.
Recommended Products
| Cat.No. | Product Name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| CDC25C-27900TH | Recombinant Human CDC25C | Human | Sf9 Insect Cell | N/A |
| CDC25C-11001H | Recombinant Human CDC25C, His-tagged | Human | E.coli | His |
| CDC25C-1576H | Recombinant Human Cell Division Cycle 25 Homolog C (S. pombe), GST-tagged | Human | Sf9 Insect Cell | GST |
| CDC25C-0918H | Recombinant Human CDC25C Protein, GST-Tagged | Human | Wheat Germ | GST |
| CDC25C-5300H | Recombinant Human CDC25C Protein, Myc/DDK-tagged, C13 and N15-labeled | Human | HEK293T | Myc/DDK |
| CDC25C-2676H | Recombinant Human CDC25C protein, His-tagged | Human | E.coli | His |
| CDC25C-3054HF | Recombinant Full Length Human CDC25C Protein, GST-tagged | Human | In Vitro Cell Free System | GST |
| CDC25C-3133M | Recombinant Mouse CDC25C Protein | Mouse | Mammalian Cell | His |
| Cdc25c-853M | Recombinant Mouse Cdc25c Protein, MYC/DDK-tagged | Mouse | HEK293T | MYC/DDK |
| CDC25C-1480M | Recombinant Mouse CDC25C Protein, His (Fc)-Avi-tagged | Mouse | HEK293 | His (Fc)-Avi |
Reference
- Liu K, et al. The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell International. 2020, 20(1): 1-16.

