What is LYZ Protein
Lysozyme, officially known as muramidase or N-acetylmuramide glycanhydrolase, is a protein that plays a pivotal role in the immune system. It is commonly referred to as LYZ, and its aliases include lysozyme C, 1,4-beta-N-acetylmuramidase, and MNLZ1. LYZ belongs to the glycoside hydrolase family 22, showcasing its enzymatic prowess in breaking down glycosidic bonds within bacterial cell walls. Structurally, it adopts a compact, globular form, with its classification falling under the alpha+beta class of proteins. Recent research has delved into the intricate details of LYZ's structure, shedding light on its dynamic nature and catalytic mechanisms.
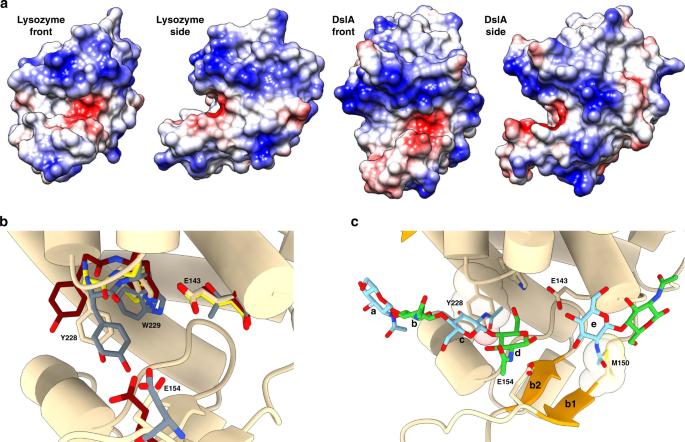
Figure 1. Active-site cleft and substrate-binding mode. (Harding C J, et al., 2020)
LYZ Biological Functions and Molecular Mechanisms
LYZ is a natural defender against bacterial invasions. Its biological functions extend beyond mere protection, encompassing the meticulous breakdown of peptidoglycan, a crucial component of bacterial cell walls. The molecular ballet performed by LYZ involves hydrolyzing the beta-1,4-glycosidic linkages in peptidoglycan, ultimately leading to bacterial lysis. This innate ability to thwart bacterial threats has positioned LYZ as a frontline guardian in our immune arsenal.
The molecular mechanisms underlying LYZ's actions are elegantly orchestrated. The protein's catalytic site, comprised of a series of amino acid residues, interacts with the peptidoglycan substrate. This interaction triggers a cascade of events, culminating in the cleavage of glycosidic bonds. The end result is the destabilization and rupture of bacterial cell walls, rendering them susceptible to the body's immune defenses.
LYZ Related Signaling Pathway
The signaling pathways associated with LYZ are intricate, reflecting the multifaceted nature of the immune response. LYZ expression is modulated by various factors, including the presence of bacterial antigens and immune mediators. Key signaling pathways, such as the NF-κB pathway, have been identified as regulators of LYZ transcription. Understanding these signal pathways not only unravels the complexities of LYZ regulation but also provides potential targets for therapeutic interventions aimed at bolstering immune defenses.
LYZ Related Diseases
While LYZ is primarily a defender, imbalances in its levels or functionality can have implications for human health. Recent studies have linked alterations in LYZ expression to certain pathological conditions. For instance, diminished LYZ activity has been associated with an increased susceptibility to bacterial infections, highlighting the crucial role this protein plays in maintaining immune homeostasis. On the flip side, elevated LYZ levels have been implicated in inflammatory disorders, suggesting a delicate equilibrium that must be maintained for optimal health.
LYZ's Applications in Biomedicine
The versatility of LYZ extends beyond its role as an immune sentinel, finding applications in various biomedical domains. In diagnostic development, LYZ has emerged as a biomarker for certain infections, with its levels serving as indicators of immune system activity. This has paved the way for the development of diagnostic assays that leverage LYZ as a reliable marker for bacterial infections.
Vaccine development has also embraced the prowess of LYZ. Researchers are exploring the inclusion of LYZ or LYZ-derived peptides in vaccine formulations to enhance the immune response against bacterial pathogens. By harnessing the natural antibacterial properties of LYZ, these vaccines aim to confer heightened protection against infections.
In the realm of therapeutics, the potential of LYZ is being harnessed for its antimicrobial properties. LYZ-based therapies are being explored as alternatives or adjuncts to traditional antibiotics, offering a targeted approach to combat bacterial infections while minimizing the risk of resistance development.
Recommended Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| LYZ-3196H | Recombinant Human LYZ protein, His-SUMO-tagged | Human | E.coli | His-SUMO |
| LYZ-4477H | Recombinant Human LYZ Protein (Lys19-Val148), His tagged | Human | E.coli | His |
| LYZ-64H | Active Recombinant Human Lysozyme | Human | Rice Grain | N/A |
| LYZ-6038HF | Recombinant Full Length Human LYZ Protein, GST-tagged | Human | In Vitro Cell Free System | GST |
| LYZ-4478H | Recombinant Human LYZ Protein (Lys19-Val148), C-His tagged | Human | Mammalian cells | C-His |
| LYZ-275H | Recombinant Human LYZ protein, His-tagged | Human | HEK293 | His |
| LYZ-2382H | Recombinant Human LYZ Protein (Lys19-Val148), His tagged | Human | E.coli | His |
| LYZ-4479H | Recombinant Human LYZ Protein (Lys19-Val148), N-His tagged | Human | E.coli | N-His |
| LYZ-1033H | Recombinant Human LYZ, MYC/DDK-tagged, 13C & 15N Labeled | Human | HEK293 | Myc/DDK |
| LYZ-2582HFL | Recombinant Full Length Human LYZ, Flag-tagged | Human | Mamanlian cells | Flag |
Reference
- Harding C J, et al. A lysozyme with altered substrate specificity facilitates prey cell exit by the periplasmic predator Bdellovibrio bacteriovorus. Nature Communications. 2020, 11(1): 4817.

