What is Neuraminidase
Neuraminidase, formally known as N-Acetylneuraminyl hydrolase, is a pivotal enzyme with a significant role in various biological processes. Commonly referred to as sialidase, this enzyme belongs to the glycoside hydrolase family 34, a group of proteins involved in the hydrolysis of glycosidic bonds. Neuraminidase operates on sialic acid residues, facilitating their removal from glycoproteins and glycolipids. This essential enzyme is a key player in the intricate dance of molecular interactions within living organisms.
Neuraminidase Structural Characteristics and Classification
Structurally, neuraminidase is characterized by a tetrameric arrangement of subunits, each possessing an active site crucial for its catalytic function. It is classified into several types based on its antigenic properties, with influenza A and B viruses being the most well-known carriers of neuraminidase. The classification system, denoted by N1 to N9 for influenza A, contributes to the understanding of viral diversity and aids in vaccine development strategies.
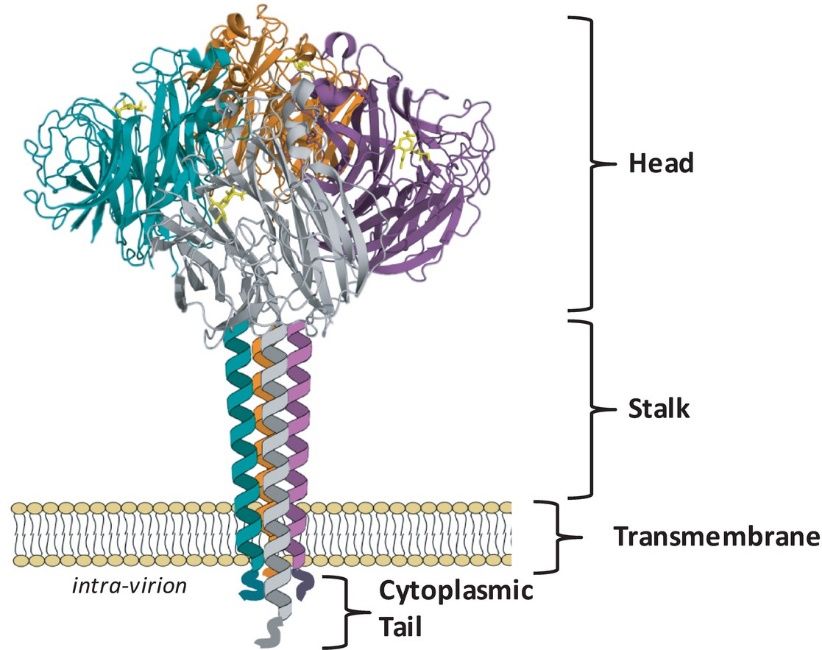
Figure 1. NA exists as a tetramer of four identical monomers. (McAuley J L, et al., 2019)
Recent Research Advances about Neuraminidase
Recent research in the field of neuraminidase has unveiled its complex interplay in various cellular processes. From deciphering its role in viral replication to exploring its involvement in cell signaling pathways, scientists are unraveling the multifaceted nature of this enzyme. Novel insights into neuraminidase's structure-function relationships are paving the way for innovative therapeutic interventions and antiviral strategies.
Neuraminidase Biological Functions and Molecular Mechanisms
At its core, neuraminidase serves as a molecular scissors, cleaving sialic acid residues from glycoconjugates. This degradative action is crucial for various physiological functions. Notably, during influenza infection, neuraminidase aids in the release of newly formed viral particles from infected cells, facilitating their spread. Additionally, neuraminidase plays a role in cell adhesion and signaling, influencing processes such as immune response and tissue development.
Neuraminidase Related Signaling Pathway
The signaling pathways associated with neuraminidase are intricate and diverse. In viral infections, neuraminidase can modulate host cell signaling, influencing immune responses and cellular processes. Moreover, neuraminidase's interaction with sialic acid receptors on cell surfaces can trigger signaling cascades, impacting cell adhesion and migration. Understanding these pathways is crucial for unraveling the broader implications of neuraminidase in health and disease.
Neuraminidase Related Diseases
Disruptions in neuraminidase function can lead to several diseases. In the context of influenza, the virus exploits neuraminidase for efficient replication and transmission. Targeting neuraminidase has become a cornerstone in antiviral drug development, aiming to curb the spread of influenza by inhibiting the enzyme's activity. Beyond influenza, neuraminidase dysfunction is implicated in genetic disorders like sialidosis, highlighting the enzyme's significance in maintaining cellular homeostasis.
Neuraminidase's Applications in Biomedicine
The significance of neuraminidase extends beyond the realm of basic research, finding practical applications in biomedical fields. In diagnostic development, neuraminidase is harnessed for its ability to cleave sialic acid residues, serving as a valuable tool in glycan analysis and biomarker detection. Furthermore, neuraminidase plays a pivotal role in vaccine development, where its inclusion ensures the production of effective and immunogenic vaccines against influenza.
In therapeutics, the inhibition of neuraminidase has become a focal point for antiviral drug development. Drugs like oseltamivir target neuraminidase, impeding the release of viral particles and reducing the severity and duration of influenza infections. The ongoing exploration of neuraminidase inhibitors holds promise for combatting other diseases with aberrant neuraminidase activity.
Recommended Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| NA-422H | Active Recombinant H1N1 NA | H1N1 | Human Cell | N/A |
| NA-309I | Recombinant H1N1 (A/California/04/2009) NA protein, His-tagged, Active | H1N1 | Insect Cell | His |
| NA-178V | Active Recombinant Influenza A H1N1(A/Puerto Rico/8/1934) NA protein | H1N1 | HEK293 | N/A |
| NA-778H | Recombinant Influenza A H1N1 (A/Michigan/45/2015) NA Protein (His36-Lys469), His-tagged | H1N1 | HEK293 | His |
| NA-421H | Recombinant H1N1 NA, His-tagged | H1N1 | Human Cell | His |
| NA-224H | Recombinant Influenza A H1N1 (A/California/04/2009) NA Protein, His-tagged | H1N1 | Baculovirus-Insect Cells | His |
| NA-484H | Recombinant H1N1 Neuraminidase Protein(Active) | H1N1 | HEK293 | N/A |
| NA-571H | Recombinant Influenza A H1N1 (A/Brisbane/02/2018) NA Protein, His-tagged | H1N1 | Insect Cells | His |
| NA-308I | Recombinant Influenza A H1N1 NA, Fc tagged | H1N1 | Human Cell | Fc |
| NA-225H | Recombinant Influenza A H1N1 (A/Michigan/45/2015) NA Protein, His-tagged | H1N1 | Baculovirus-Insect Cells | His |
Reference
- McAuley J L, et al. Influenza virus neuraminidase structure and functions. Frontiers in Microbiology. 2019, 10: 39.

