What is TFRC Protein
The Transferrin Receptor Protein 1 (TFRC/CD71), a ubiquitously expressed type II transmembrane protein, discovery did not happen overnight but was the result of years of meticulous and relentless research. In the 1980s, researchers recognized it as transferrin receptor, an integral membrane glycoprotein found in the plasma membrane, which binds and internalizes iron-loaded transferrin. This discovery pushed the envelope for further investigation into the physiological and pathological roles of TFRC.
Gene locus and protein structure
Located on chromosome 3q29, the TFRC gene is comprised of 20 coding exons spanning approximately 27,023 bases. It encodes the TFRC protein, which has a molecular weight of about 95 kilodaltons. Structurally, it is composed of three distinct domains - a cytoplasmic domain, a transmembrane domain, and an extracellular domain. Featuring a homodimeric structure, each chain of the dimer is connected via a helix to form a binding pocket for iron-loaded transferrin.
Function of TFRC protein
The primary function of TFRC protein is iron homeostasis. The iron absorbed by the duodenum and packed into transferrin is transported in the bloodstream. The TFRC proteins, located in the cell membrane, capture these transferrin-iron complexes, internalizing and breaking them down to release iron within the cells. Apart from essential metabolite transportation, TFRC proteins also function in cell growth and proliferation as the requirement for iron increases during these processes.
TFRC protein-related signal pathway
The function of TFRC is crucial in certain signal pathways, particularly the JAK-STAT pathway. The intertwined mechanisms of iron homeostasis and erythropoiesis involve the central participation of the transferrin receptor. The binding of erythropoietin (EPO) to its receptor (EPOR) induces JAK2 - a non-receptor tyrosine kinase - to trigger the STAT5 pathway. Activated STAT5 enhances the transcription of TFRC gene leading to an increase of the transferrin receptor, hence augmenting iron uptake for hemoglobin production.
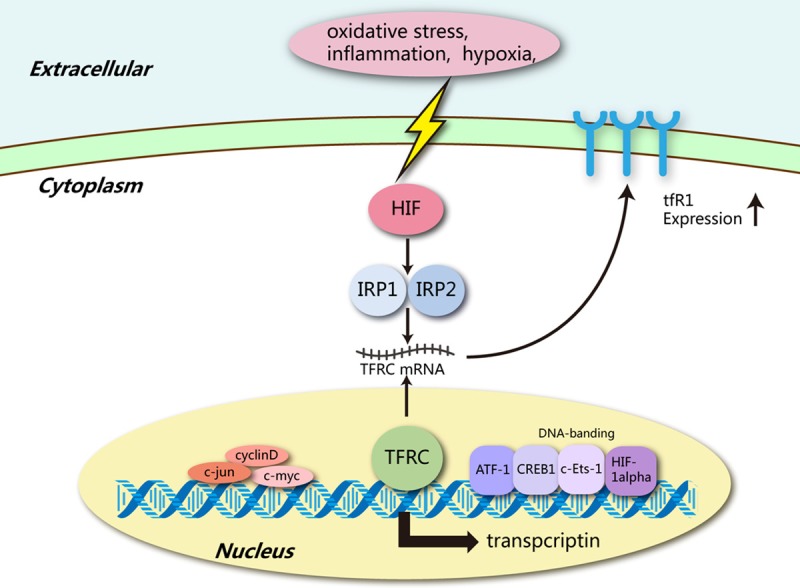
Fig1. Transferrin receptor 1 in cancer. (Shen Y, et al. 2018)
TFRC protein-related diseases
Imbalances in TFRC protein functioning can trigger various diseases, majorly relating to iron metabolism. Iron-overload conditions, such as Hemochromatosis and iron-loading anaemias like Beta-Thalassemia and Sideroblastic anemia, are associated with augmented TFRC protein activity. Conversely, iron deficiency-related diseases like Anemia of Chronic Disease entails reduced TFRC activity. Apart from metabolic conditions, the upregulation of TFRC protein is observed in numerous cancers owing to the high metabolic rate of cancerous cells necessitating increased iron uptake.
Applications in Biomedical
The extensive knowledge of TFRC proteins has been leveraged in the biomedical industry for both diagnostic and therapeutic purposes. They act as potential biomarkers for early disease detection, especially in cancer diagnostics. Therapeutically, antibody-drug conjugates targeting TFRC proteins are utilized in cancer treatment. Additionally, CRISPR-Cas9 gene-editing technology exploits the conspicuously high expression of TFRC protein in cancer cells.
To conclude, TFRC proteins, while minuscule in size, carry significant responsibility for iron homeostasis in the human body. While simplistic in their primary function of iron transportation, their complex structure, involvement in vital signal pathways, and association with various diseases lead to a deeper understanding of the remarkable intricacies of cellular functioning. Thus, TFRC proteins, with their newly-discovered trail of biotechnological applications, have emerged as powerful agents in the arsenal of molecular biology and medicine.
Our Featured Products
| Cat.No. | Product Name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| TFRC-3864H | Recombinant Human TFRC protein, His-tagged, Biotinylated | Human | HEK293 | His |
| Tfrc-5164M | Recombinant Mouse Tfrc, His tagged | Human | HEK293 | His |
| TFRC-3865H | Active Recombinant Human TFRC Protein, His-tagged, Biotinylated | Human | HEK293 | His |
| TFRC-705H | Recombinant Human TFRC Protein, MYC/DDK-tagged | Human | HEK293 | Myc/DDK |
| TFRC-2029H | Recombinant Human TFRC Protein, His-Avi-tagged | Human | HEK293 | His-Avi |
| TFRC-12H | Recombinant Human TFRC Protein (101-760aa), C-His-tagged | Human | Insect Cell | C-His |
| TFRC-2179H | Recombinant Human TFRC Protein, His (Fc)-Avi-tagged | Human | HEK293 | His-Avi |
| TFRC-466H | Recombinant Human TFRC Protein, His-tagged, Biotinylated | Human | HEK293 | His |
| Tfrc-01M | Recombinant Marmoset TFRC Protein, His-tagged | Marmoset | HEK293 | His |
Reference
- Shen Y, Li X, Dong D, et al. Transferrin receptor 1 in cancer: a new sight for cancer therapy. American Journal of Cancer Research. 2018 ;8(6):916-931. PMID: 30034931; PMCID: PMC6048407.

