Principle and Protocol of Co-Immunoprecipitation
Co-Immunoprecipitation (Co-IP) was developed from the immunoprecipitation technique with which Co-IP shares the fundamental principle of the specific antigen-antibody reaction. Immunoprecipitation(IP) technique is used for isolate individual protein. Using an antibody that is specific for a particular protein, the target protein could be isolated out from a crude lysate of a plant or animal tissue or other biological regent.
Immunoprecipitation of intact protein complexes is known as co-IP, which could pull the entire protein complex out of solution and thereby identify unknown members of the complex. Co-IP is a powerful technique that is used regularly by molecular biologists to analyze protein–protein interactions.
Prepare lysate form cells or tissue samples which express the target protein is the first step of the co-IP. In order to preserve the intact of protein-protein interactions, the lysis buffers should use non-ionic detergents (e.g., NP-40, Triton X-100). Then the target proteins are captured by specific antibodies from total lysate. The resultant immunocomplexes (composed of antibody, protein of interest (antigen), and antigen-associated proteins) can be precipitated using a resin (e.g., agarose, sepharose, or magnetic beads) that is conjugated with IgG-binding Protein A/G. The third step is washes. Irrelevant, non-binding proteins, antigens and any proteins that are bound are eluted by series of washes. Then, the bound proteins which eluted are analyzed by SDS-PAGE/immunoblotting and/or mass spectrometry.
As mentioned above, proper experimental conditions must be determined for each protein-protein interaction. Selection of an optimal lysis buffer and immunoprecipitation antibody are the two most important aspects for the success of a co-IP experiment. To overcome these problem, the protein of interest is often fused with an epitope tag (e.g., flag, myc, HA, his, V5), or a fluorescence protein (e.g., GFP, DsRed) at the terminus of the protein, and ectopically expressed in cells. Antibodies for these “tags” that are compatible with IP/co-IP have been well developed and are commercially available from multiple manufacturers.
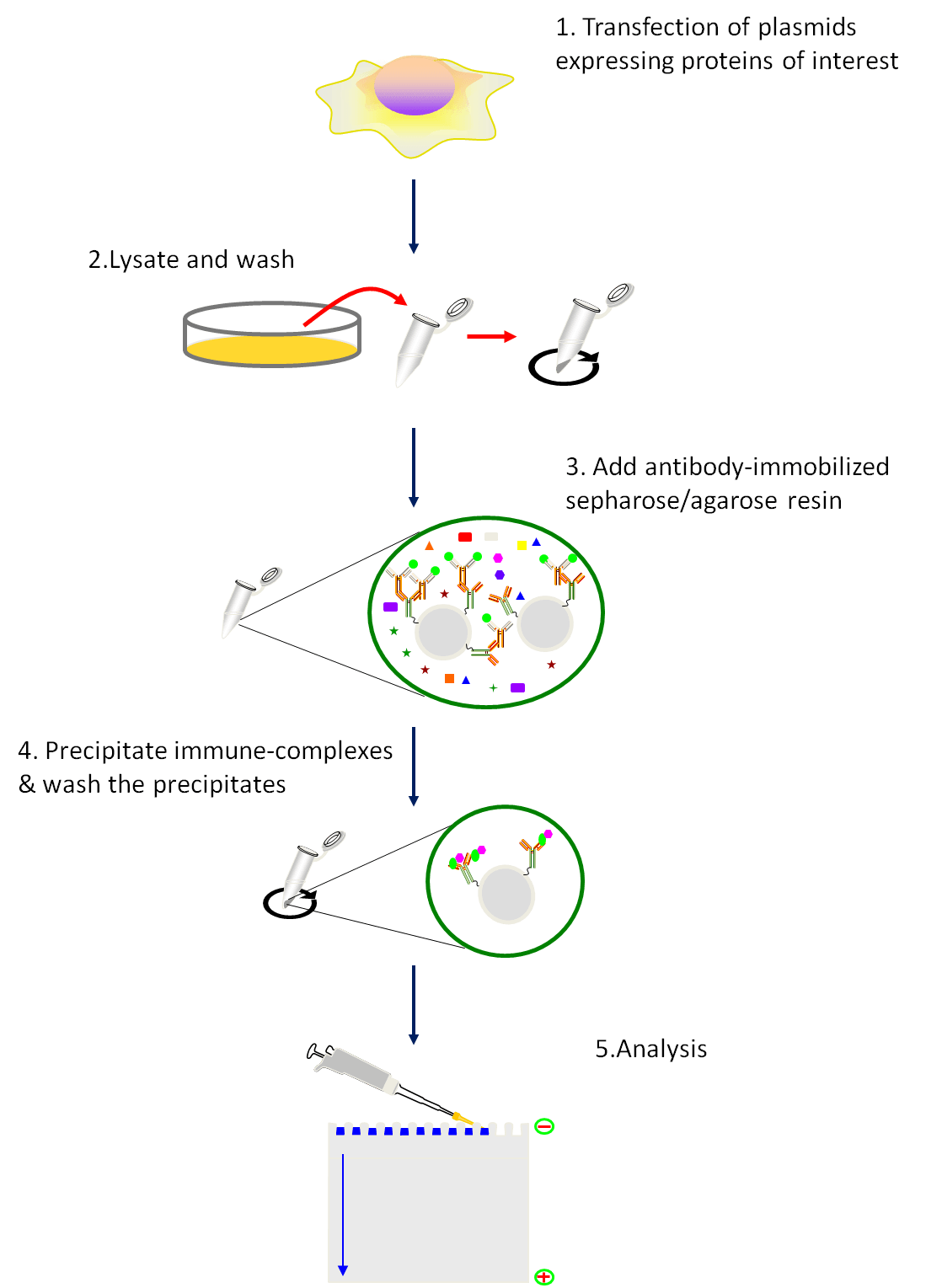 Fig1. Schematic of the co-immunoprecipitation procedure.
Fig1. Schematic of the co-immunoprecipitation procedure.
Procedure:
- Transfection of plasmids expressing proteins of interest. Follow the transfection protocol.
- 48h after tansfection, harvest the cells using a cell scraper and pellet the cell by centrifugation at 1000g×6min at 4℃.
- Resuspend the cells tenderly by pre-cold PBS. Centrifugation at 1000g×6min at 4℃ and discard the supernatant.
- Repeat Step 3 for three times.
- Resuspend the cells tenderly by pre-cold lysis buffer (add protease and phosphatase inhibitor prior to use).
- Incubate the samples on ice for 30 min. Convert the tube up and down during this period.
- Centrifuge at 15,000 g×10 min at 4℃ and transfer the supernatant to a new 1.5 ml tube.
- Measure protein concentration.
- Using a tip to transfer the IgG-crosslinked resins to a EP tube containing 1ml PBS.
- Pellet the resin by centrifugation at 6000 g×30 s, and aspirate the supernatant.
- Repeat step 10 for three times.
- Resuspend the resin in pre-cold lysis buffer.
- Transfer 750μg of total protein to a new tube and adjust the volume to 200μl with pre-cold lysis buffer.
- Add 50μl of the prepared resin.
- Incubate the tubes on a tube rotator at a slow speed for 1 h at 4 ℃.
- Centrifugation at 6000 g for 30 s at 4 ℃ and transfer 200 μl of the supernatant to a new tube. Save the pelleted resins to use as negative controls.
- Dilute the antibody to proper concentration with lysis buffer.
- Add diluted antibody to lysate prepared in step 16 and incubate the tube on a rotation 1h (or overnight) at 4 ℃.
- Prepare Protein G-immobilized resin as instruction of step 9-12. (Protein G is often considered a more universal IgG binding protein than is Protein A, but different species, and subtypes of species, do vary in their binding to these proteins.)
- Add 50 μl of the prepared resin to each reaction tube in step 18 and incubate on a rotator for 1h at 4 ℃.
- Centrifugation at 6000g×30 s at 4 ℃. Transfer 100 μl of supernatant to a new tube and aspirate the remaining supernatant.
- Resuspend the resin with 500 μl pre-cold lysis buffer, centrifugation at 6000g×30 s at 4 ℃, and and aspirate the remaining supernatant.
- Repeat step 22 for three times.
- Resuspend the resin-bound immune complexes in 20 μl of 2 Laemmli buffer, boil for 5 min, and analyze by SDS-PAGE/immunoblot analysis.
We will be glad to discuss details of intended interaction studies with you and develop experimental strategies/methods tailored to your requirement. Please see co-IP service in our web and get in contact with Creative BioMart.
Welcome to contact us or send an email to for project quotations and more detailed information.

