Principle and Protocol of Kjeldahl Method
Protein content determination method is one of the most common and basic analysis methods in biochemical research. Kjeldahl determination of nitrogen is a commonly used method for analyzing nitrogen content of organic compounds at present. It is one of the most accurate and simple methods for determining total organic nitrogen in samples, and has been taken as the standard inspection method determined internationally and domestically. Kjeldahl method has wide application scope, accurate determination results and good reproducibility, but the operation is complex, time-consuming and reagent consumption is large. If the modular digestion furnace is used instead of the traditional digestion device, several samples can be determined at the same time, which can save time and improve work efficiency. It is suitable for the determination of batch protein, and has the advantages of accuracy, speed, simplicity, low consumption, stability, etc. Although Kjeldahl method is more complex, it is more accurate. The protein determined by Kjeldahl method is often used as the standard protein of other methods. The optimum digestion conditions of Kjeldahl method samples are affected by the dosage of copper sulfate, potassium sulfate and concentrated sulfuric acid. Among them, copper sulfate is the first major factor, and the amount of potassium sulfate and concentrated sulfuric acid is the second and third major factors.
Protein is a nitrogen containing organic matter. The nitrogen content of protein in nature is relatively stable, that is, the average nitrogen content of 100g protein is 16g. Therefore, when determining the protein content of biological samples, the nitrogen content in the samples can be measured first, and then multiplied by the coefficient 6.25, which can be converted into protein content. The determination of protein by Kjeldahl method can be divided into four processes: sample digestion, distillation, absorption and titration. The principle is that the nitrogenous organic compounds in the sample are co digested with concentrated sulfuric acid under the action of CuSO4 catalyst and K2SO4 which improves the boiling point of the solution. The nitrogenous organic compounds decompose to produce ammonia, which in turn reacts with sulfuric acid to become ammonium sulfate. Then add alkali and distill to release ammonia. The ammonia is absorbed with excessive boric acid solution. Then titrate with hydrochloric acid standard solution to obtain the total nitrogen and convert it into protein content. The chemical reaction process is as follows:
1. Under the action of strong heat, CuSO4 and concentrated H2SO4, nitrogen in organic matter is digested to form (NH4)2SO4 with the reaction formula as follows:
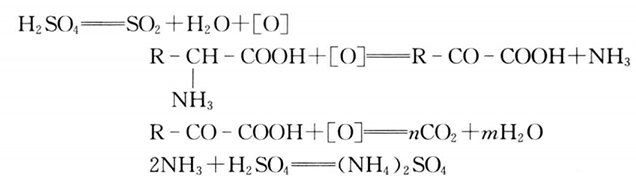
2. It acts with alkali in Kjeldahl nitrogen determiner, releases NH3 through distillation, and collects it in H3BO3 solution, with the reaction formula as follows:
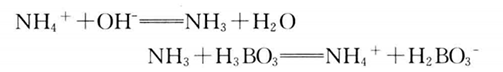
3. Titrate with HCl standard solution of known concentration, calculate the nitrogen content according to the HCl consumption, and then multiply by the corresponding conversion factor to obtain the protein content reaction equation:
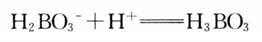
1. Main Instruments and Equipment
Digestive tube or Kirschner flask, Kjeldahl nitrogen determination distillation device, alcohol lamp, 100mL conical flask, 100ml measuring cylinder, watch glass, acid burette, small funnel, glass bead, etc.
2. Experimental Materials
Protein sample to be tested.
3. Main reagents
Digestive solution: the proportion of 30% hydrogen peroxide, sulfuric acid and water is 3:2:1, which is prepared before use.
Catalyst: grind copper sulfate (CuSO4 · 5H2O) and potassium sulfate (K2SO4) in 1:3 ratio to mix 50% sodium hydroxide solution.
2% boric acid solution.
Standard hydrochloric acid solution (about 0.01mol/L).
Mixed indicator (Tian's indicator) *1: it is prepared by mixing 50mL 0.1% bromocresol green ethanol solution and 200mL 0.1% methyl red ethanol solution, and stored in a brown bottle for use.
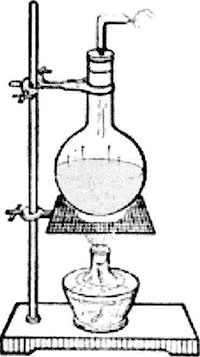
1. Installation of Micro Kjeldahl Nitrogen Analyzer (Figure 2-1-6) *2
The nitrogen determination instrument is composed of steam generator, reaction chamber and condenser tube. The steam generator includes a heat source and a 3-5L flask *3. The sample and alkali liquor are directly poured into the reaction chamber from the small beaker on the upper side of the reaction chamber. There is a long glass tube in the center of the reaction chamber, with the upper end leading to the outer layer of the reaction chamber and the lower end close to the bottom of the reaction chamber. An opening is arranged at the bottom of the lower end of the reaction chamber, and a rubber tube and a spiral clamp are arranged on the opening. The reaction waste liquid is discharged. The nitrogen generated by the reaction can be passed into the collecting bottle through the thin tube at the upper end of the reaction chamber through the condensation tube. The reaction chamber and the condensation pipe are connected by a rubber pipe.
When installing the instrument, fix the steam generator vertically on the iron stand, and connect the steam generator, reaction chamber and condensate pipe with rubber pipes. The rubber pipe shall be connected at the same horizontal position. The distance between the lower end of the condenser tube and the test bench shall be subject to the availability of the collecting bottle. After installation, it shall not be moved easily to avoid damage to the instrument. Carefully check the whole device for air leakage to ensure the accuracy of the measured results.
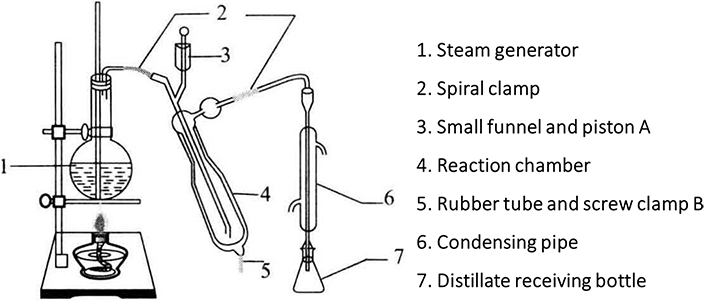 Figure 2-1-6 Installation Diagram of Kjeldahl Nitrogen Analyzer
Figure 2-1-6 Installation Diagram of Kjeldahl Nitrogen Analyzer
2. Nitrification of Sample
(1) Accurately weigh 100-500 mg (depending on the nitrogen content in the sample) of dry powder of plant sample (passing 40-60 mesh sieve) on the analytical balance, 2 portions. (2) Take three 50mL Kjeldahl flasks, and send the weighed samples to the bottom of the first and second flasks respectively. The third flasks do not add samples as blank controls to determine the trace nitrogen compounds in the reagents. Add 0.3g of catalyst and 5mL *4 of concentrated sulfuric acid into each flask, and then place the flask on the nitrification rack of the fume hood. Heat the flask with low fire first. After the water in the flask has evaporated, the sulfuric acid starts to emit SO2 white smoke. Increase the flame to keep the liquid slightly boiling until the solution is blue green, clear and transparent *5. Cool it and wait for distillation.
3. Ammonia Distillation
(1) Washing of distillation unit: first, clean the Kjeldahl nitrogen analyzer with tap water, and then install it according to Figure 2-1-6. Add about 2/3 of the volume of distilled water into the steam generator (flask 1), add a few drops of concentrated sulfuric acid (under acidic conditions, ammonia in the water will not be distilled out) and zeolite to make the boiling even, close piston A and spiral clip B, boil the water in flask 1, fully wash the instrument with steam, and check whether there is air leakage in all parts of the device, then open piston A, let the steam washing funnel for about 5min, and then close piston A, Continue distilling for about 5min to wash all distillers. First remove the triangular flask, then remove the alcohol lamp, clamp the spiral clamp 2 between the steam generator and the reaction chamber, discharge the reaction waste liquid and flush the small beaker, open the spiral clamp B and piston A, and the waste liquid is discharged from the bottom of the rubber tube 5. After the distillation of each sample, the above washing process must be carried out for 2-3 times.
(2) Distillation of digestive solution: first open the piston A and spiral clip B, and then put the prepared collecting bottle containing 5mL 2% boric acid and 2 drops of mixed indicator at the lower end of the condenser tube. The nozzle of the condenser pipe shall be immersed under the liquid level. Take 1mL of distilled water and add it to the Kjeldahl flask to mix it with the nitrating solution. Carefully pour this solution into the reaction chamber 4 from the funnel, and then wash the Kjeldahl flask with a small amount of distilled water. The washing solution is also poured into the reaction chamber 4 from the funnel, and washed for three times in this way. Then add 30% NaOH 7mL from the funnel, wash the funnel twice *6 with a little distilled water, close the piston A and screw clip B, immediately heat the flask 1 to boil, and start distillation. The liquid in the collecting bottle changes from brownish purple to blue-green, and then continues to steam for another 3min. Lower the collecting bottle so that the condensing nozzle is about 1cm away from the liquid level, and continue to distill for 1min. Flush the lower end of the condenser tube with a small amount of distilled water, and the washing liquid flows directly into the collection bottle, then remove the collection bottle, and then move the alcohol lamp, open the screw clamp B and piston A to discharge the waste liquid. Then wash the distiller according to the above steps for standby.
4. Titration
After distillation, titrate the solution in the collecting bottle with 0.01mol/L hydrochloric acid to change the solution from blue-green to lavender, which is the end point. Record the volume (mL) of 0.01 mol/L hydrochloric acid consumed by the sample and blank control respectively, expressed in VS and V0.
5. Calculation
Calculate the protein content in the sample according to the following formula: X (g/100g) = [ (VS - V0) × c × 0.014/m] × F × 100 VS is the volume of hydrochloric acid used to titrate the sample (mL). V0 is the volume of hydrochloric acid used for titration blank (mL). c is the volume of standard hydrochloric acid concentration (mL). 0.014 is the mass (g) of nitrogen equivalent to 1.000mol/L hydrochloric acid standard solution. m is the sample mass (mg). F=6.25 is the average value of nitrogen protein conversion coefficient. Different materials have different conversion coefficients. Rice flour is 5.95, wheat is 5.7, barley is 5.8, rye is 5.8, oats is 5.7, and corn is 5.9.
1. This method is applicable to the detection of semi-solid samples and liquid samples. The general sampling range of semi-solid samples is 2.00-5.00g. Sample 10.0-25.0mL of liquid sample (equivalent to 30-40mg of nitrogen). If the liquid sample is tested, the result is expressed in g/100mL.
2. During nitration, if the sample contains high sugar or high fat, pay attention to control the heating temperature to avoid a large amount of foam blowing out of the Kjeldahl flask, causing loss of the sample. A small amount of octanol or liquid paraffin, or silica nitrate foaming agent can be added to reduce the production of foam.
3. When nitrating, pay attention to rotating the Kjeldahl flask, flushing the carbon particles attached to the bottle wall, and nitrating the sample thoroughly. If the sample is not easy to nitrify to be clear and transparent, cool the solution in the Kjeldahl flask, add a few drops of hydrogen peroxide, and then continue heating to nitrify completely.
4. The temperature of boric acid absorption solution should not exceed 40 °C, or the ammonia absorption will weaken, resulting in low detection results. The collecting bottle can be placed in a cold bath.
5. The absolute difference between two independent measurement results obtained under repeatability conditions shall not exceed 10% of the arithmetic mean.
*1 This indicator is purplish red in acid and green in alkaline, with a narrow and sensitive discoloration range.
*2 At present, there are various commercial full-automatic nitrogen determinators, which are easy to use.
*3 Simple nitrogen determination device.
*4 Be careful when adding. Concentrated sulfuric acid is highly corrosive. Pay attention to protection.
*5 During the nitrification process, the flask shall be rotated frequently to make the carbonized particles on the bottle wall nitrify completely. To shorten the nitrification time, 3-4 drops of 30% hydrogen peroxide can be added when the solution turns light brown.
*6 Each time the liquid is discharged, the piston A shall be controlled, and a little distilled water shall be kept in the funnel to prevent ammonia from escaping.

