Protocol of the Detection of ³²P Radioactive Markers
The 32P radiolabeling assay is the most classic method for detecting phosphorylated proteins. The combination of 32P radiolabeling and bi-directional gel electrophoresis allows for a holistic view of intracellular protein phosphorylation from a proteomic perspective, and the sensitive and visual detection of phosphorylated proteins is the reason why this method is still widely used today. However, this method suffers from radioactive contamination and cannot label tissue samples.
In this section, we use sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by radioautography to detect the labeled phosphorylated proteins. 32P radiolabeling assays are performed by treating cells with 32P-labeled ATP, incorporating 32P into the phosphorylated proteins, and finally detecting them.
In this section, the detection of 32P radiolabeled suspension cells is explained as an example.
The purpose of this lab manual is to help researchers master the basic principles and main steps of 32P radiolabeling detection of phosphorylated proteins and understand the application of 32P radiolabeling detection of phosphorylated proteins method.
Protein phosphorylation is a process catalyzed by a series of protein kinases that transfer the γ-phosphate of ATP to a target site.32Pradiolabeled assays are now commonly used to detect phosphorylated proteins. Phosphorylation of proteins in living cells can usually be detected in the presence of radiolabeled orthophosphate. The commonly used 32P is a beta-ray radiation source, and because this beta-ray radiation source is low energy, strict P is being used more and more widely. Phosphate is taken up by cells and converted to labeled ATP by the nucleic acid synthesis pathway. Cells cannot be labeled with radioactive ATP because ATP cannot be transported across the cell membrane. After SDS-PAGE, labeled proteins are exposed by radioactive autoradiography (-70°C) to enhance the screen, which can increase the sensitivity of the assay or be detected by phosphorescent imager.
SDS-PAGE is the introduction of SDS into a polyacrylamide gel system. SDS breaks intra- and intermolecular hydrogen bonds and disrupts the secondary and tertiary structure of proteins, forming negatively charged SDS-protein complexes that reduce or eliminate the natural charge differences between various protein molecules, and the migration rate of protein molecules depends on the size of the molecule when performing electrophoresis. In SDS-PAGE discontinuous electrophoresis, the gel-making buffer used is the Tris-HCl buffer system, the concentrated gel buffer pH 6.7, and the separation gel buffer pH 8.9; while the electrophoresis buffer used is the Tris-glycine buffer system. The protein samples were concentrated in the concentrated gel and then entered the separation gel, and then passed through the molecular sieve effect of the separation gel and separated under the electric field according to the size of the relative molecular mass of the proteins.
1. Main Instruments and Equipment
Micropipettes, Petri dishes, glass rods, incubators, centrifuges, SDS-PAGE electrophoresis devices, radioactive autoradiography devices, radioactive 32P protection devices, films, exposure devices.
2. Material
The cell culture to be labeled, H332PO4.
3. Main reagents
(1) Phosphorus-free medium.
(2) SDS gel spiking buffer:
| 50mmol/L | Tris-HCl (pH 6.8) |
| 100 mmol/L | DTT |
| 2% | SDS |
| 10% | Glycerol |
| 0.1% | Bromophenol Blue |
(3) Separation buffer.
(4) Concentrated buffer.
(5) 30% gel storage solution (Acr: Bis=29:1).
(6) 10% SDS solution.
(7) Electrophoresis buffer (Tris-glycine buffer, pH8.3).
(1) Phosphoric acid can be labeled in phosphate-free medium and can be purchased from different companies.
(2) The cells to be labeled were cultured in phosphorus-free medium to the maximum density*1.
(3) Take the cultured cells, gently centrifuge 2000g for 1min, suspend the cells with standard medium, gently centrifuge 2000g for 1min, and discard the supernatant.
(4) Cells were diluted with complete medium containing 10% phosphate to the appropriate concentration and added to a suitable sized culture dish*2 at an amount of 2 mL × 107 cells per 2 mL, labeled between 0.1 μCi and 1 mCi/10 cm dish*3, and incubated for 2 h.
(5) After incubation, the cells were collected and centrifuged at 2000g for 1 min, and the supernatant was discarded.
(6) Add lysate to collect proteins: the precipitate was suspended in 100μL 1×SDS gel spiking buffer.
(7) Heat at 100°C for 3min, centrifuge at high speed at room temperature for 1min, and place on ice for assay.
(8) SDS-PAGE test.
① Wash the glass plate of electrophoresis tank, blow it dry, seal the edge with Vaseline, install the glue preparation device, and prepare 12% separation glue *4.
② Pour the prepared separation gel into the gel tank immediately to 2/3 of the height of the gel tank, and seal it with distilled water.
③ After the glue is solidified, it takes about 30min to pour out the water and absorb it with absorbent paper.
④ Prepare 5% concentrated gel*4, and pour the prepared concentrated gel into the gel tank (it is better to insert the comb without overflowing).
⑤ Insert the comb and pull it out after the glue has solidified. Put it into the electrophoresis tank, and add the prepared sample into the sample hole.
⑥ Connect the electrophoresis instrument, turn on the power, adjust the voltage to 80V, adjust the voltage to 120V after the sample runs through the concentrated gel (about 30min), and stop electrophoresis when the indicator (/pomophenol blue) reaches 1cm from the bottom edge of the gel, which takes about 2h, and the electrophoresis is over.
⑦ The sensitivity of the measurement can be increased by enhancing the screen exposure by detecting the signal with the phosphorescent imager. The preparation method of glue is shown in the following table.
| 12% Separation gel*4 | 12% Concentrated gel*4 | |
| H2O | 2.45mL | 2.05mL |
| 30% Acrylamide | 3.0mL | 0.5mL |
| Tris buffer | 1.9mL (1.5mol/L,pH8.8) | 375μL (1.0mol/L,pH 6.8) |
| 10%SDS | 75μL | 30μL |
| 10% Ammonium persulfate *5 | 75μL | 30μL |
| TEMED*6 | 3μL | 3μL |
Notes.
Acrylamide is a nerve agent, be careful when using it, please make sure to wear gloves when handling it.
Ammonium persulfate needs to be prepared fresh and pay attention to the concentration, otherwise it will affect the gum solidification.
1 piece of gum can be mixed with 10ml. centrifuge tube, 2 pieces of gum should be mixed using a small triangular bottle, the residual liquid after preparation can be temporarily kept for reference gum solidification time, generally 30min can be.
The test results are shown in Figure 17-1-1.
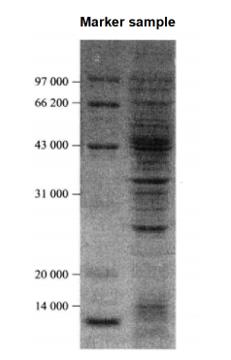 Figure 7-1-1 SDS-PAGE electrophoresis graph to examine the results of 32P labeling assay of suspended cells
Figure 7-1-1 SDS-PAGE electrophoresis graph to examine the results of 32P labeling assay of suspended cells
1. The concentration and growth status of cultured suspension cells have a great impact on labeling efficiency, and attention should be paid to mastering various growth conditions of cells during operation.
2. Radioactive marking operation shall be carried out with protective measures.
3. During SDS-PAGE detection, the concentration of polyacrylamide gel shall be properly adjusted according to the relative molecular weight of protein.
4. Note that poor sample dissolution or excessive gel concentration will cause tailing of electrophoresis bands.
*1 Generally, it takes 2-3 days to culture, depending on the cell concentration.
*2 Pay attention to all operations behind the protective device.
*3 In the initial experiment, 500 Ci/100 mm dishes are recommended.
*4 The concentration can be adjusted appropriately according to the protein characteristics.
*5 It needs to be prepared fresh and added before filling.
*6 Add before glue filling.

