Human Cell Line Activation Test (h-CLAT)
The Human Cell Line Activation Test (h-CLAT) is a widely accepted non-animal method used to assess the skin sensitization potential of chemicals. This assay measures changes in the expression of cell surface markers associated with dendritic cell activation and supports the evaluation of the third key event of the skin sensitization Adverse Outcome Pathway (AOP). At Creative BioMart, we provide a fully validated, GLP-compliant h-CLAT service using THP-1 human monocytic leukemia cells to quantify CD86 and CD54 expression following exposure to test substances. Our service delivers reliable concentration-response data that help classify sensitization potential and support regulatory submissions with high confidence.
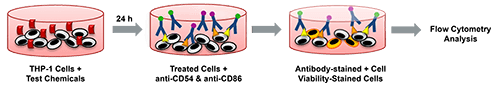
Understanding Human Cell Line Activation Test (h-CLAT)
Skin sensitization refers to the immunological process in which repeated exposure to a chemical leads to sensitization of skin tissue, ultimately provoking allergic contact dermatitis (ACD). This condition, characterized by inflammation, redness, and discomfort, represents a significant public health concern and is closely monitored in industries such as cosmetics, personal care, household chemicals, agrichemicals, and pharmaceuticals. As consumers increasingly demand safe formulations and as global regulatory bodies tighten their requirements, accurate sensitization assessment has become essential in early-stage product development.
Historically, animal models—most notably the Local Lymph Node Assay (LLNA) and Guinea Pig Maximization Test (GPMT)—were the standard methods for skin sensitization testing. However, social, regulatory, and ethical pressures have driven a shift toward non-animal approaches. European Union regulations, including the Cosmetics Directive and REACH, have accelerated this transition by prohibiting animal testing for cosmetic ingredients and favoring alternative testing methods.
Among the suite of validated in vitro assays, the Human Cell Line Activation Test (h-CLAT) provides a powerful tool to evaluate the activation of dendritic cells, a critical event in the sensitization AOP. Dendritic cells serve as antigen-presenting cells that initiate T-cell–mediated immune responses. When chemicals interact with the skin, sensitizers trigger phenotypic changes in these cells, including upregulation of co-stimulatory molecules such as CD86 and adhesion molecules like CD54.
The h-CLAT assay recreates this immune activation using the THP-1 human monocytic leukemia cell line, which exhibits dendritic-cell-like behavior when exposed to allergens. By quantifying CD86 and CD54 expression using flow cytometry, the assay provides mechanistic evidence of sensitizing potential. It also generates concentration-dependent activation curves that contribute to potency ranking, an increasingly important requirement under modern hazard assessment frameworks.
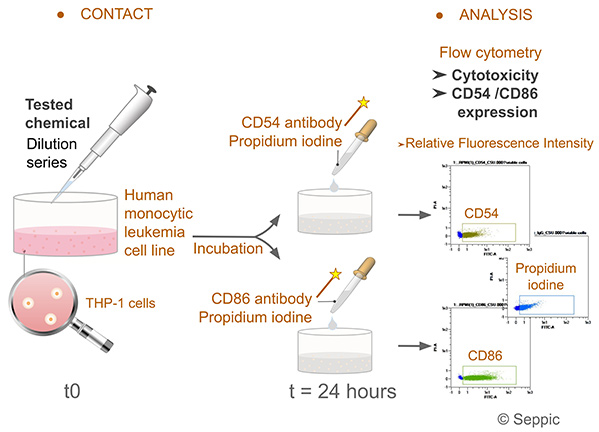
Figure 1. Human Cell Line Activation Test (h-CLAT) protocol according to OECD 442E. (Puginier et al., 2022)
Despite its strong predictive ability—particularly for extreme, strong, and moderate sensitizers—the h-CLAT should be used as part of an integrated testing strategy due to the complexity of immune-mediated skin sensitization. When paired with assays addressing earlier AOP key events, such as KeratinoSens™ (Nrf2 activation) or DPRA (peptide reactivity), h-CLAT contributes to a comprehensive weight-of-evidence assessment. Creative BioMart provides all three assay types, enabling a streamlined and harmonized sensitization testing workflow for customers across industries.
Human Cell Line Activation Test (h-CLAT): What We Offer
Creative BioMart delivers a high-quality, GLP-compliant h-CLAT testing solution tailored to the needs of research teams, product developers, and regulatory affairs professionals. Our offering includes:
- Validated h-CLAT workflow following current OECD testing principles and industry standards.
- Use of THP-1 cells as a representative human cell model for dendritic-cell activation.
- Quantitative measurement of CD86 and CD54 surface marker expression using calibrated flow cytometry.
- Comprehensive concentration-response data, including replicates for robust statistical interpretation.
- Clear sensitization predictions using the established Relative Fluorescence Intensity (RFI) criteria.
- Expert interpretation, integrating assay outcomes with broader AOP-based skin sensitization frameworks.
- Regulatory-ready reporting for submissions under REACH, CLP, cosmetics regulations, and internal R&D risk assessments.
Supported Applications
Our services support diverse application needs, including:
- Hazard identification for raw materials and ingredients
- Screening of new chemical entities (NCEs) for early-stage toxicity profiling
- Replacement of historical animal data in regulatory dossiers
- Comparative assessment of structurally related analogs or formulation variants
- Prioritization of compound libraries in product development pipelines
Service Workflow
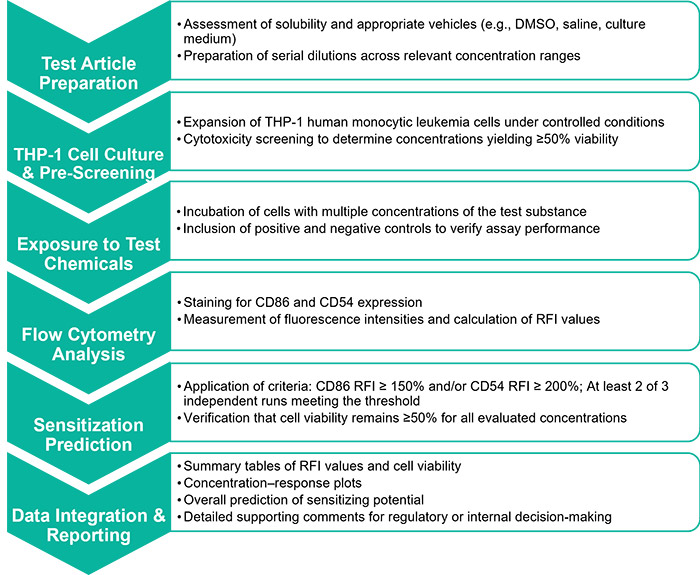
Assay Model and Biological Basis
The h-CLAT addresses Key Event 3 of the skin sensitization AOP: dendritic cell activation. THP-1 cells respond to potential sensitizers by upregulating CD86 and CD54—markers critical for T-cell co-stimulation and antigen presentation. These changes are quantifiable and provide insight into the immunogenic potential of chemicals.
Prediction Model
Positive Result:
- CD86 RFI ≥ 150% and/or
- CD54 RFI ≥ 200%
- Valid in 2 out of 3 independent experiments
Viability Criterion:
- Cell viability must be ≥50%, as measured by Propidium Iodide exclusion
This prediction model has been supported across multiple validation studies and is recognized for its high accuracy in identifying strong and extreme sensitizers.
Test Chemical Compatibility
The h-CLAT is suitable for a broad range of substances, including:
What Sets Us Apart
- GLP-Compliant, Internally Validated h-CLAT Platform: Our facility adheres to Good Laboratory Practice standards, ensuring data integrity, traceability, and regulatory acceptance. The assay has been fully validated internally to match international guidelines.
- High Sensitivity for Moderate to Strong Sensitizers: Our optimized THP-1 culture system, calibrated flow cytometry, and strict QC controls enhance detection accuracy across a range of sensitizer potencies.
- Experienced Scientific Team: Our toxicologists and immunology specialists have extensive experience with the AOP framework, helping clients interpret results and integrate h-CLAT outcomes with DPRA and KeratinoSens™ data.
- Flexible Project Design: We accommodate custom exposure setups, vehicle options, extended concentration ranges, and integrated multi-assay packages tailored to your development stage.
- Fast Turnaround and Transparent Communication: Clients receive frequent updates, clear expectations, and precise scheduling. Our streamlined workflow supports rapid screening and iterative formulation refinement.
- Comprehensive Reporting for Regulatory Submission: Our reports meet the expectations of global regulatory bodies and include detailed experimental conditions, QC criteria, raw data, statistical analyses, and interpretive commentary.
Human Cell Line Activation Test (h-CLAT): Case Studies
Case 1: Inter-laboratory evaluation of h-CLAT for in vitro sensitization testing
Sakaguchi et al., 2006. doi:10.1016/j.tiv.2005.10.014
This case study assesses the performance of the Human Cell Line Activation Test (h-CLAT) using THP-1 and U-937 cells as alternatives to traditional skin sensitization assays. After protocol optimization, two laboratories tested nine chemicals and measured CD86 and CD54 expression following 24- and 48-hour exposures at multiple IC50-based concentrations. THP-1 cells showed higher accuracy than U-937 cells for distinguishing allergens from non-allergens, with 24-hour treatments performing best. CD86 and CD54 were strong predictive markers in THP-1, while CD86 alone performed better in U-937. Both labs achieved consistent results, supporting h-CLAT—particularly THP-1 at 24 hours—as a reliable in vitro sensitization model.
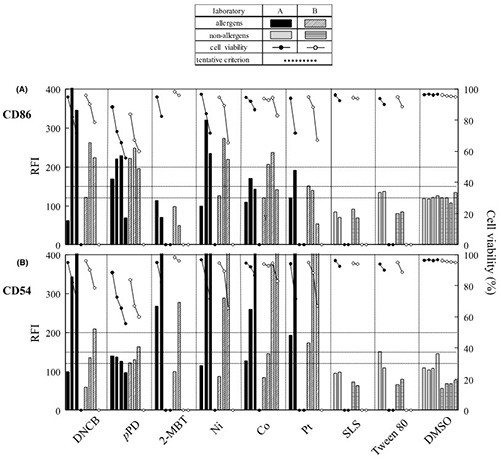
Figure 2. The effect of chemical allergens and non-allergens on THP-1 cell expression of CD86 (A) and CD54 (B) for both laboratories at 24 h treatment. The cells were treated with nine chemicals at four concentrations. After the treatment, the expression of CD86 (A) and CD54 (B) for both laboratories at 24 h treatment. The cells were treated with nine chemicals at four concentrations. After the treatment, the expression of CD86 (A) and CD54 (B) was measured by flow cytometry and RFIs were calculated. The lines across the graph indicate RFI values of 120, 150 and 200. (Sakaguchi et al., 2006)
Case 2: Predicting sensitizer potency using h-CLAT metrics
Nukada et al., 2012. doi:10.1016/j.tiv.2012.07.001
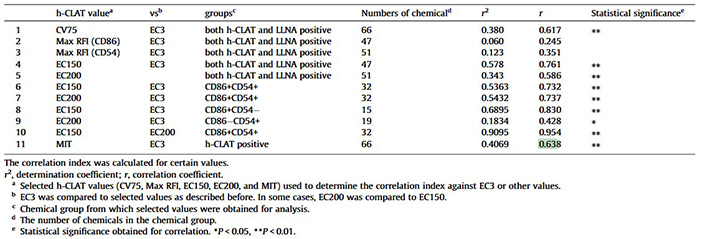
This case study evaluates whether the Human Cell Line Activation Test (h-CLAT) can predict skin-sensitizing potency by comparing its metrics to LLNA EC3 values. h-CLAT measures CD86 and CD54 upregulation in THP-1 cells, and several derived parameters—CV75, EC150, and EC200—showed significant correlation with LLNA potency estimates. From EC150 and EC200, a minimum induction threshold (MIT) was defined, which correlated with EC3 (R = 0.638) and achieved roughly 80% accuracy for GHS sub-categorization using a 13 µg/mL cutoff. These findings suggest that h-CLAT metrics, particularly MIT, offer a useful approach for estimating allergenic potency.
Table 1. Results of the statistical analysis for the comparison between h-CLAT values and LLNA EC3. (Nukada et al., 2012)
Human Cell Line Activation Test (h-CLAT): Customer Feedback
"We partnered with Creative BioMart to evaluate a new series of preservative candidates for our skincare formulations. Their h-CLAT service provided clear and reproducible CD86 and CD54 induction data, allowing us to differentiate moderate and strong sensitizers from non-sensitizers. The comprehensive concentration-response curves and viability assessments were particularly useful in supporting our internal safety review. Their team’s guidance on integrating the h-CLAT data with DPRA and KeratinoSens™ results made our weight-of-evidence analysis much more straightforward."
— Senior Toxicology Manager | Global Cosmetics Company
"Our R&D team needed rapid and reliable non-animal sensitization data for several industrial intermediates. Creative BioMart’s h-CLAT testing not only adhered to GLP standards but also delivered robust RFI measurements across multiple concentrations. The predictive accuracy for strong and moderate sensitizers gave us confidence in early hazard classification, helping us prioritize safer compounds for scale-up. The detailed report was well-structured and suitable for regulatory submission under REACH guidelines."
— Director of Safety Assessment | Multinational Chemical Manufacturer
"During the development of a topical drug formulation, we required h-CLAT data to assess the sensitization potential of a novel excipient. Creative BioMart’s team performed the assay efficiently, providing detailed CD86/CD54 induction profiles and cell viability data. The results helped us make informed decisions on excipient selection and supported our safety dossier for regulatory review. Their responsiveness and scientific expertise made a significant difference in accelerating our formulation optimization process."
— Head of Preclinical Development | Mid-Size Pharmaceutical Company
"We engaged Creative BioMart to test a series of new surfactant blends for a sensitive-skin detergent line. Their h-CLAT service delivered high-quality data showing RFI values well below the positive thresholds, confirming a low sensitization risk. The team also provided interpretive comments that helped our regulatory group contextualize the findings within the broader AOP framework. The fast turnaround and clear communication allowed us to confidently move forward with our product launch schedule."
— Product Safety Lead | Household & Personal Care Brand
Human Cell Line Activation Test (h-CLAT) Service: Frequently Asked Questions
-
Q: What types of substances can be tested using h-CLAT?
A: Our h-CLAT service is suitable for a wide range of chemicals, including cosmetic ingredients, household chemicals, pharmaceuticals, industrial intermediates, preservatives, and novel formulation excipients. The assay can also accommodate low- and high-solubility compounds following appropriate vehicle optimization. -
Q: How does h-CLAT work and what does it measure?
A: The assay measures dendritic-cell-like THP-1 human monocytic cells for phenotypic changes induced by sensitizers. Specifically, it quantifies surface expression of CD86 and CD54 via flow cytometry. Increased expression of these markers indicates dendritic cell activation, which is a critical key event in the skin sensitization Adverse Outcome Pathway (AOP). -
Q: Can h-CLAT detect weak sensitizers?
A: Yes, the assay can detect weak sensitizers, though the detection rate is lower compared to moderate and strong sensitizers. We recommend using h-CLAT in combination with other complementary assays (e.g., KeratinoSens™ or DPRA) to enhance predictive accuracy across all potency ranges. -
Q: Is your h-CLAT service GLP-compliant?
A: Absolutely. Creative BioMart’s h-CLAT service follows Good Laboratory Practice (GLP) standards, ensuring reproducibility, data integrity, and regulatory acceptance. All experimental steps, including THP-1 culture, chemical exposure, flow cytometry, and data reporting, are fully traceable and validated internally. -
Q: How fast is the h-CLAT testing process?
A: Our streamlined workflow allows rapid turnaround while maintaining quality. From THP-1 cell preparation to final data delivery, the standard assay can be completed efficiently, making it suitable for both early-stage screening and regulatory support. -
Q: Can your team help integrate h-CLAT data with other non-animal sensitization tests?
A: Yes. Creative BioMart offers integrated testing strategies combining h-CLAT with KeratinoSens™ and DPRA assays. This approach covers multiple key events in the AOP, enabling a robust weight-of-evidence assessment for sensitization potential and regulatory submission readiness.
Other Resources
Related Services
References:
- Nukada Y, Ashikaga T, Miyazawa M, et al. Prediction of skin sensitization potency of chemicals by human Cell Line Activation Test (h-CLAT) and an attempt at classifying skin sensitization potency. Toxicology in Vitro. 2012;26(7):1150-1160. doi:10.1016/j.tiv.2012.07.001
- Puginier M, Roso A, Groux H, Gerbeix C, Cottrez F. Strategy to avoid skin sensitization: application to botanical cosmetic ingredients. Cosmetics. 2022;9(2):40. doi:10.3390/cosmetics9020040
- Sakaguchi H, Ashikaga T, Miyazawa M, et al. Development of an in vitro skin sensitization test using human cell lines; human Cell Line Activation Test (h-CLAT) II. An inter-laboratory study of the h-CLAT. Toxicology in Vitro. 2006;20(5):774-784. doi:10.1016/j.tiv.2005.10.014
- Wong CL, Ghassabian S, Smith MT, Lam AL. In vitro methods for hazard assessment of industrial chemicals – opportunities and challenges. Front Pharmacol. 2015;6. doi:10.3389/fphar.2015.00094
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

