KeratinoSens™ Assay
Evaluating the skin sensitization potential of chemicals is essential for ensuring consumer safety and meeting global regulatory requirements. The KeratinoSens™ assay is an EURL ECVAM–validated, cell-based reporter gene test that quantifies the activation of the Nrf2–ARE signaling pathway in response to electrophilic compounds. By measuring luciferase induction following exposure to test substances, this in vitro assay provides a predictive and reproducible method for assessing the second key event of the skin sensitization Adverse Outcome Pathway (AOP). Creative BioMart offers a highly standardized, fully transferable KeratinoSens™ assay service, supporting researchers across cosmetics, chemicals, pharmaceuticals, and related industries in generating high-quality, regulatory-compliant sensitization data.
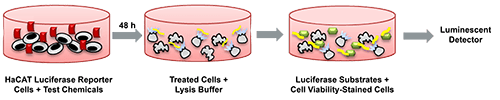
Background: What Is KeratinoSens™ Assay
Skin sensitization is a complex immunological process in which repeated exposure to a substance triggers an allergic response, often manifesting clinically as allergic contact dermatitis (ACD) . For decades, the evaluation of sensitizers relied heavily on animal-based assays such as the Local Lymph Node Assay (LLNA) and guinea pig tests. However, following regulatory shifts—particularly in the cosmetic sector—non-animal alternatives have become both necessary and scientifically preferred.
One of the most important advances in non-animal toxicology is the development of mechanistically informed assays aligned with the Adverse Outcome Pathway (AOP) for skin sensitization. The KeratinoSens™ assay addresses Key Event 2: Keratinocyte Activation, specifically the activation of the Nrf2–Keap1–ARE pathway, a critical cellular defense mechanism against electrophilic and oxidative stress.
The assay is built upon a stable human keratinocyte cell line engineered to express luciferase under the control of an antioxidant response element (ARE) derived from the promoter region of the human AKR1C2 gene. This gene, known to be upregulated by sensitizers, serves as a reliable biological marker for Nrf2 pathway activation.
Upon exposure to sensitizing chemicals, Nrf2 dissociates from Keap1 and translocates into the nucleus to induce ARE-regulated genes. The consequent increase in luciferase activity provides a quantifiable readout of sensitization potential.
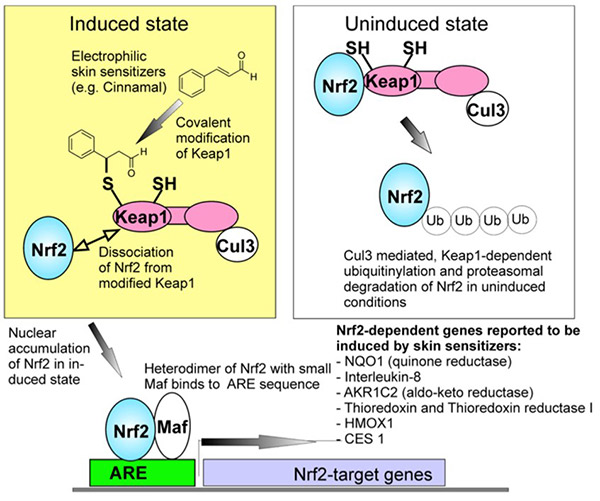
Figure 1. A general view of the induction of the Nrf2 pathway by skin sensitizers. (Natsch, 2010)
The KeratinoSens™ assay stands out for:
- High mechanistic relevance, directly measuring a key event of the AOP
- Quantitative, dose-dependent output based on luciferase induction
- Validated performance, recognized by EURL ECVAM and OECD TG 442D
- Direct applicability to personal care, household products, agrichemicals, and pharmaceuticals
Creative BioMart’s robust platform ensures accurate, reproducible results while offering flexible study designs to address diverse testing needs.
KeratinoSens™ assay: What We Offer
Creative BioMart provides a complete, turnkey KeratinoSens™ assay service that includes:
-
Assay Implementation with Validated Procedures
We follow OECD TG 442D and EURL ECVAM–validated protocols using a licensed KeratinoSens™ cell line to ensure regulatory-accepted outcomes.
-
Comprehensive Dose–Response Evaluation
Each test substance is evaluated across 12 serial concentrations, allowing us to calculate:
- EC1.5
- MEC (Minimum Effective Concentration)
- AC₅₀
- IC₃₀
- Maximum response ( Iₘₐₓ )
-
Dual-Endpoint Measurement
We assess both:
- Cell viability (cytotoxicity)
- Luciferase induction (Nrf2–ARE activation)
This dual-parameter approach supports mechanistic accuracy and helps interpret borderline or ambiguous results.
-
Flexible Sample Handling
We accommodate test substances of various solubilities and formats, optimizing vehicle selection (e.g., DMSO) to maintain cellular integrity and assay performance.
-
High-Quality Reporting
Clients receive complete data packages, including:
- Dose–response curves
- Statistical significance analysis
- Sensitization potential classification
- Raw and processed data
- Method documentation and QC performance metrics
-
Expert Consultation
Our scientists support every stage—from study design and dose range justification to data interpretation and regulatory alignment.
Service Workflow
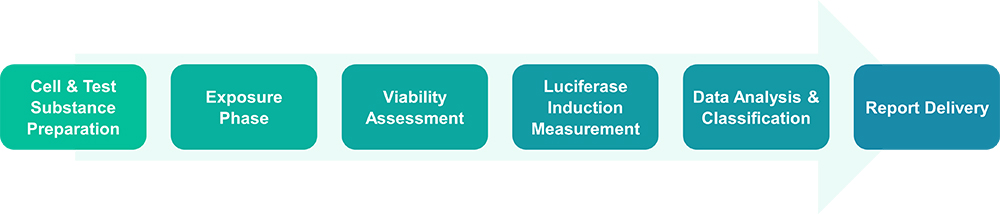
KeratinoSens™ Assay Protocol
- Grow cells in 96-well plates for 24 hours.
- Dissolve test chemicals in DMSO.
- Add chemicals to cells at 12 graded concentrations.
- Incubate for 48 hours.
- Measure cell viability.
- Assess luciferase activity by lysing cells, adding luciferin substrate, and recording light output.
- A compound is considered positive when luciferase induction exceeds 1.5-fold with statistical significance.
Service Features
Creative BioMart’s KeratinoSens™ platform is designed to support a wide range of applications and sample types:
| Features | |
|---|---|
|
Industries Served |
|
|
Assay Strengths |
|
|
Regulatory Alignment |
Our assay is compliant with:
Safety evaluation frameworks under EU REACH, EPA, DEFRA, and cosmetic regulations |
|
Additional Capabilities |
When used together with other non-animal assays such as DPRA and h-CLAT, the KeratinoSens™ assay provides strong weight-of-evidence for hazard classification. |
What Sets Us Apart
- Strict Compliance with Validated Protocols: We implement the KeratinoSens™ assay exactly as defined by OECD TG 442D, ensuring regulatory-acceptable results.
- High Reproducibility and Predictive Accuracy: Our facilities use optimized culture conditions, calibrated luminometry, and validated dose ranges to guarantee consistent and reliable performance.
- Comprehensive Data Interpretation: Our scientific team provides expert insights into EC1.5 values, cytotoxicity profiles, borderline outcomes, and sensitizer classification.
- Flexible Testing Options: We support single-compound studies, high-throughput screening of chemical libraries, and customized exposure conditions upon request.
- Fast Turnaround and Competitive Pricing: Our streamlined workflow delivers high-quality results quickly, making the assay ideal for early-stage decision-making or formulation refinement.
- Broad Industry Experience: With extensive project history in cosmetics, household chemicals, agrochemicals, and pharmaceuticals, we understand diverse regulatory expectations and provide tailored reporting formats.
KeratinoSens™ Assay: Case Studies
Case 1: The sensitivity of the KeratinoSens™ assay to evaluate plant extracts: A pilot study
Andres et al., 2013. doi:10.1016/j.tiv.2013.02.008
The KeratinoSens™ assay was evaluated as a tool for identifying sensitizing components in botanical cosmetic mixtures, which are more complex than the pure substances used in traditional validation. Four plant extracts were spiked with graded levels of citral, cinnamic aldehyde, and isoeugenol. While unspiked extracts tested negative, most spiked samples became positive, enabling estimation of the minimum detectable sensitizer concentration. Sensitizers at ≥2% were reliably identified, though high cytotoxicity in one extract reduced assay sensitivity. Overall, the study demonstrates proof-of-concept that KeratinoSens™ can assess mixtures and detect low-level sensitizing ingredients within complex botanical matrices.
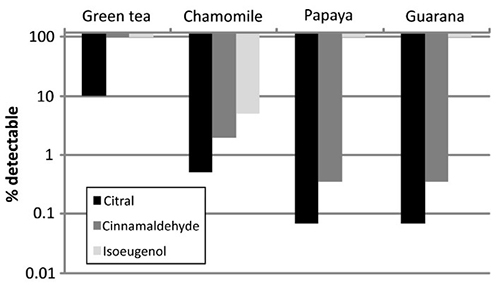
Figure 2. Minimal detection level in % for citral, cinnamaldehyde and isoeugenol in each botanical mixture. (Andres et al., 2013)
Case 2: Impact of irritants on allergen sensitization thresholds in the KeratinoSens™ assay
De Rentiis et al., 2021. doi:10.1111/cod.13762
This study used the KeratinoSens™ assay to examine how irritants influence the sensitization thresholds of allergens within mixtures, reflecting real-world exposures that often trigger contact dermatitis. A moderate (cinnamal) and a weak (ethylene glycol dimethacrylate) allergen were first tested individually, then combined with noncytotoxic levels of three irritants—sodium dodecyl sulfate, salicylic acid, and α-pinene. Mixture testing revealed lower sensitization thresholds compared with single-substance assessments, with shifts ranging from 1.1- to 10.3-fold. Despite these changes, the allergen remained the dominant driver of the response. The findings highlight how irritants can modulate allergen potency in the KeratinoSens™ assay.
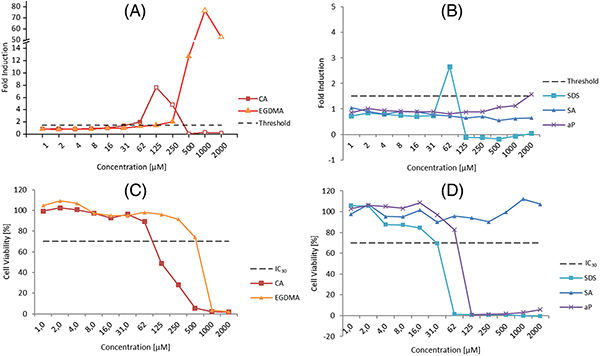
Figure 3. The maximal induction factors ( Iₘₐₓ )of the luciferase activities and the cell viability curves for (A,C) sensitizers and (B,D) nonsensitizers. aP, α-pinene; EGDMA, ethylene glycol dimethacrylate; IC30, concentration at which 30% reduction of cell viability occurs; SA, salicylic acid; SDS, sodium dodecyl sulfate. (De Rentiis et al., 2021)
KeratinoSens™ Assay: Customer Feedback
"We relied on Creative BioMart’s KeratinoSens™ assay to screen a new series of bioactive fragrance molecules before entering formulation stability studies. Their team delivered EC1.5 and AC50 values with exceptional clarity, and the dose–response curves were among the cleanest we’ve seen from any external CRO. The rapid turnaround allowed us to eliminate two high-risk candidates early and move forward confidently with our lead blend. Their scientific guidance on integrating KeratinoSens™ with DPRA data was an added bonus that helped streamline our internal risk assessment workflows."
— Senior Toxicology Manager | Global Cosmetics Company
"For our new polymer additive program, we required dependable non-animal sensitization data to support an internal go/no-go decision. Creative BioMart’s KeratinoSens™ service exceeded expectations. Their adherence to the validated EURL ECVAM protocol, coupled with precise luciferase quantification across 12 concentrations, gave us highly reproducible results—even for our low-solubility test article. The team proactively communicated throughout the process, and the final report aligned seamlessly with our OECD TG 442D documentation needs."
— Director of Safety Assessment | Multinational Chemical Manufacturer
"We engaged Creative BioMart to evaluate several early-stage immunomodulatory compounds that presented borderline sensitization signals in our preliminary screens. Their KeratinoSens™ assay provided definitive EC1.5 thresholds and Iₘₐₓ values, enabling us to differentiate true sensitizers from noise. The scientists took the time to walk us through subtle curve characteristics, which was invaluable for our AOP-based decision tree. Their professionalism and deep understanding of Nrf2/ARE pathway biology made a noticeable difference in the quality of the results."
— Head of Preclinical Development | Mid-Size Pharmaceutical Company
"Our team partnered with Creative BioMart to test a new surfactant system intended for a sensitive-skin detergent line. Because the formula contained multiple electrophilic intermediates, we needed a reliable KeratinoSens™ readout to predict consumer safety. Creative BioMart delivered a comprehensive data package—including viability plots, EC1.5 values, and maximum luciferase induction—that our regulatory group described as ‘submission ready.’ Their efficient 48-hour exposure workflow and transparent data QC allowed us to accelerate our launch timeline by several weeks."
— Product Innovation Lead | Household & Personal Care Brand
KeratinoSens™ Assay Service: Frequently Asked Questions
-
Q: What types of compounds can be evaluated using your KeratinoSens™ assay?
A: Our KeratinoSens™ platform supports a broad range of substances, including cosmetic and personal-care ingredients, specialty chemicals, household formulations, agrochemical active ingredients, and early-stage pharmaceutical compounds. The assay is highly adaptable and designed for both early screening and regulatory-oriented studies. -
Q: How reliable and regulatory-aligned is your KeratinoSens™ service?
A: We follow the fully validated EURL ECVAM protocol, ensuring regulatory acceptance and high reproducibility. Our data packages include all standard endpoints—EC1.5, AC₅₀, Iₘₐₓ, MEC, IC₃₀, and viability curves—making them suitable for integration into OECD TG 442D-aligned submissions or broader weight-of-evidence frameworks. -
Q: What advantages does your assay design offer compared to other service providers?
A: We maintain stringent quality control across cell handling, DMSO solubilization, exposure conditions, and luciferase readouts. Each compound is tested across 12 concentrations, enabling precise dose-response modeling and reducing false positives or negatives. Our optimized workflow also minimizes assay drift and increases signal stability, ideal for challenging or borderline sensitizers. -
Q: How long does the KeratinoSens™ testing process take?
A: Our streamlined protocol—24-hour cell seeding, 48-hour exposure, and rapid luciferase/viability quantification—allows efficient turnaround. Most projects are completed within a short study window, enabling clients to make timely decisions during formulation optimization or hazard evaluation. -
Q: Can your team help integrate KeratinoSens™ results with other non-animal sensitization assays (e.g., DPRA, h-CLAT )?
A: Absolutely. Our experts routinely assist companies in building multi-assay testing strategies that follow the adverse outcome pathway (AOP) for skin sensitization. Combining KeratinoSens™ with DPRA or cellular activation assays increases predictive accuracy and supports regulatory compliance for both investigative and confirmatory testing. -
Q: Is your KeratinoSens™ assay suitable for high-throughput screening?
A: Yes. With established workflows on 96-well formats, automated liquid handling, and robust luciferase detection systems, we readily accommodate multi-compound screens—ideal for ingredient libraries, formulation candidates, and structure-activity studies.
Other Resources
Related Services
References:
- Andres E, Sá-Rocha VM, Barrichello C, Haupt T, Ellis G, Natsch A. The sensitivity of the KeratinoSensTM assay to evaluate plant extracts: A pilot study. Toxicology in Vitro. 2013;27(4):1220-1225. doi:10.1016/j.tiv.2013.02.008
- De Rentiis AMA, Pink M, Verma N, Schmitz‐Spanke S. Assessment of the different skin sensitization potentials of irritants and allergens as single substances and in combination using the KeratinoSens assay. Contact Dermatitis. 2021;84(5):317-325. doi:10.1111/cod.13762
- Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers—functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicological Sciences. 2010;113(2):284-292. doi:10.1093/toxsci/kfp228
- Wong CL, Ghassabian S, Smith MT, Lam AL. In vitro methods for hazard assessment of industrial chemicals – opportunities and challenges. Front Pharmacol. 2015;6. doi:10.3389/fphar.2015.00094
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

