Primordial and Mature Germ Cells
Creative BioMart Primordial and Mature Germ Cells Product List
Immunology Background
Background
Primordial Germ Cells
Primordial germ cells (PGCs) are the earliest precursor cells of the germ cell lineage. They are a unique population of cells that are set aside early in development and are responsible for giving rise to gametes (sperm and egg cells). PGCs are initially undifferentiated and are crucial for the transmission of genetic information to the next generation. These cells undergo a series of developmental processes to eventually differentiate into mature sperm or egg cells.
Functional Role of Primordial Germ Cells
- Gamete Formation: Primordial germ cells (PGCs) are the precursors of gametes, the reproductive cells responsible for sexual reproduction. PGCs give rise to mature sperm cells in males and egg cells in females through a series of developmental processes.
- Genetic Continuity: PGCs play a crucial role in maintaining genetic continuity across generations. They carry genetic information that is passed on to offspring, ensuring the transmission of hereditary traits.
- Genetic Diversity: Through processes like meiosis and genetic recombination, PGCs contribute to genetic diversity within a population. This diversity is essential for the adaptation and evolution of species over time.
- Reproductive Function: The primary function of PGCs is to generate mature gametes that are necessary for sexual reproduction. These gametes combine during fertilization to form a zygote, which develops into a new organism.
- Regulation of Development: PGCs influence the development of the reproductive system and related structures by interacting with surrounding cells and tissues. They are involved in the establishment of the gonadal primordia and the differentiation of germ cells into mature gametes.
Mature Germ Cells
Mature germ cells are the fully differentiated cells that develop from primordial germ cells. In males, mature germ cells develop into sperm cells through a process called spermatogenesis. In females, mature germ cells develop into ova (egg cells) through oogenesis. These cells are specialized for sexual reproduction and carry half of the genetic information required for the formation of a new individual. They are involved in the process of fertilization, where genetic material from the sperm and egg combine to form a zygote, which eventually develops into a new organism.
Functional Role of Mature Germ Cells
- Fertilization: Mature germ cells, such as sperm and egg cells, are essential for the process of fertilization. Sperm cells carry genetic material from the male, while egg cells contain genetic material from the female. Fertilization involves the fusion of these gametes to form a zygote.
- Genetic Contribution: Mature germ cells contribute genetic material to the zygote, which contains a unique combination of genetic information from both parents. This genetic contribution determines the traits and characteristics of the offspring.
- Embryonic Development: After fertilization, the zygote undergoes cell division and differentiation to form an embryo. The genetic material provided by the mature germ cells guides the development of the embryo and determines its growth and differentiation.
- Transmission of Genetic Information: Mature germ cells pass on genetic information from one generation to the next. The genetic material carried by sperm and egg cells is inherited by the offspring, ensuring the continuation of the species.
- Reproductive Potential: Mature germ cells are specialized for reproduction and have the potential to generate new life. Sperm cells are motile and capable of fertilizing an egg cell, while egg cells contain the necessary resources for supporting embryonic development after fertilization.
Developmental Processes of Primordial and Mature Germ Cells
Germ cells are specialized cells that give rise to gametes—sperm and eggs—in sexually reproducing organisms. Their development is a complex process that involves several stages, from the formation of primordial germ cells (PGCs) to the maturation of gametes. This process is crucial for sexual reproduction, genetic diversity, and the continuation of species.
Developmental Processes of Primordial Germ Cells (PGCs)
| 1. Origin and Formation |
Development Stage: PGCs are typically specified early in embryogenesis, usually during the blastocyst stage in mammals. Specification: In many organisms, PGCs arise from the epiblast and are identified by specific markers (e.g., Blimp1 in mice). Migration: After specification, PGCs migrate to the developing gonads. This migration is influenced by various signaling pathways, including BMP and Wnt signaling. |
| 2. Proliferation and Differentiation |
Proliferation: Once they reach the gonadal region, PGCs undergo extensive proliferation. This ensures a sufficient pool of germ cells for later differentiation. Epigenetic Reprogramming: PGCs undergo epigenetic changes, such as demethylation of DNA and reprogramming of histones, preparing them for the next stage of development. |
| 3. Establishment of Gonadal Territory |
Depending on the sex of the organism, PGCs will differentiate into either spermatogonia (in males) or oogonia (in females) once they have integrated into the gonads. This process is heavily influenced by the surrounding somatic cells that help induce germ cell differentiation through environmental cues and signaling molecules. |
Developmental Processes of Mature Germ Cells
| 1. Spermatogenesis (Males) |
The development from spermatogonia to mature sperm involves several stages:
|
| 2. Oogenesis (Females) |
Oogenesis is the formation of oocytes and is characterized by:
|
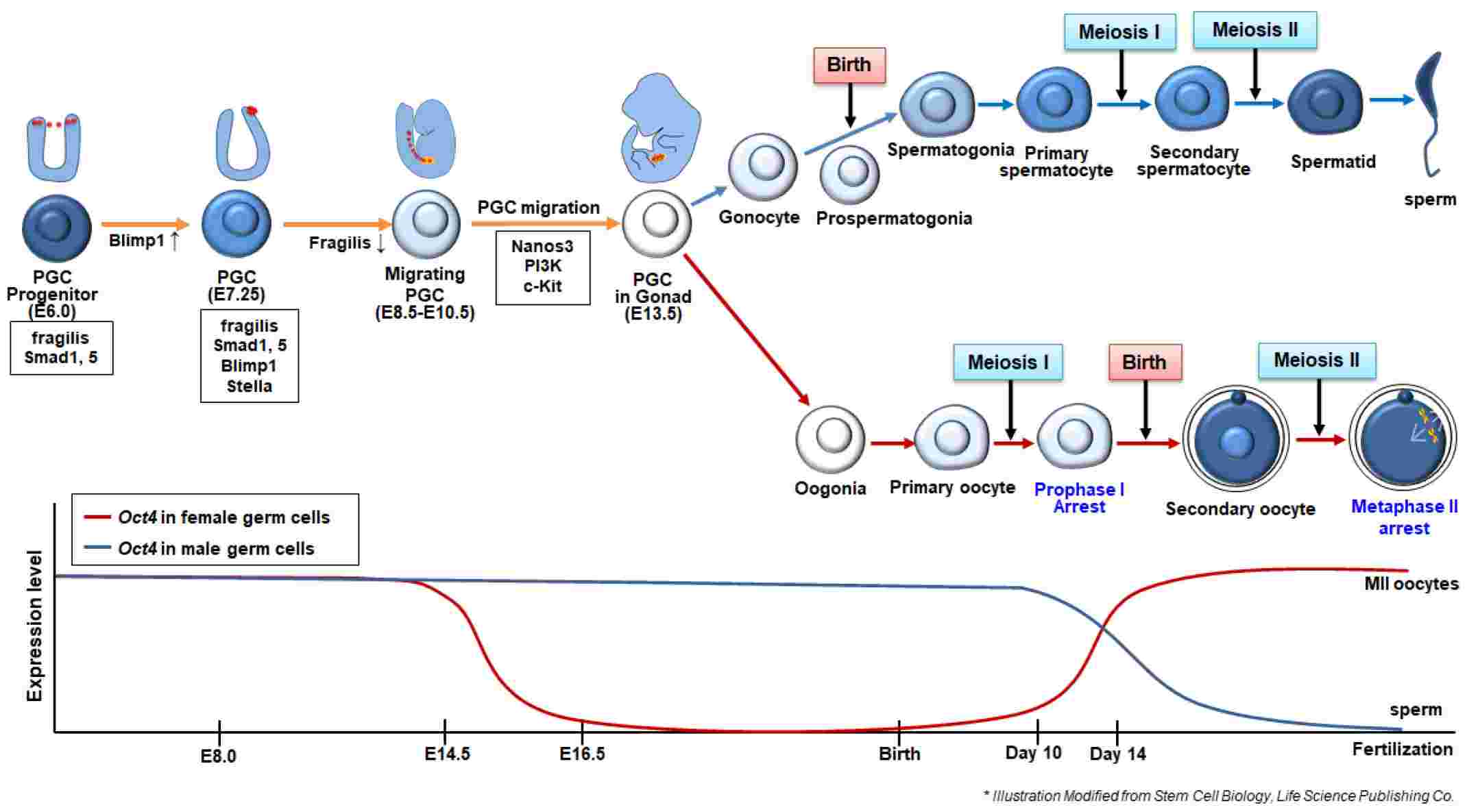 Fig.1 Summary of in vivo developmental process for the formation of male and female germ cells and the expression of germ cell-related genes during the germ cell development. (Hong T-K, et al., 2021)
Fig.1 Summary of in vivo developmental process for the formation of male and female germ cells and the expression of germ cell-related genes during the germ cell development. (Hong T-K, et al., 2021)Key Primordial and Mature Germ Cell Markers
Primordial and mature germ cell markers are proteins that are expressed on the surface of germ cells at different stages of development. These markers are used by researchers to identify and study the different stages of germ cell development, including primordial germ cells, which are the earliest progenitors of germ cells, and mature germ cells, which are fully differentiated gametes.
Some common primordial germ cell markers include Oct4, Sox2, and Nanog, which are transcription factors that are important for the maintenance of pluripotency in embryonic stem cells. These markers are also expressed in primordial germ cells and are used to identify and isolate these cells for further study.
Mature germ cell markers, on the other hand, are proteins that are specific to the differentiation and maturation of germ cells into sperm or eggs. For example, in males, markers such as DAZL, VASA, and SCP3 are commonly used to identify and study mature sperm cells, while in females, markers such as ZP3, GDF9, and BMP15 are used to identify and study mature egg cells.
| Markers | Details |
|---|---|
| Markers of Primordial Germ Cells |
|
| Markers of Mature Germ Cells |
|
Overall, primordial and mature germ cell markers play a crucial role in understanding the development and function of germ cells, and they are essential tools for researchers studying reproductive biology and fertility.
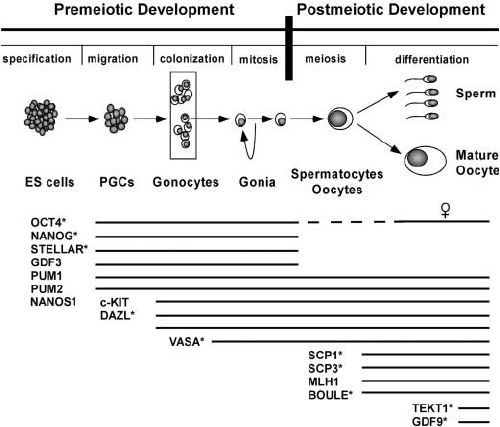 Fig.2 Schematic representation of the different stages and their markers of germ cell differentiation and their markers during fetal and adult development. (Clark AT, 2004)
Fig.2 Schematic representation of the different stages and their markers of germ cell differentiation and their markers during fetal and adult development. (Clark AT, 2004)Future Research Directions and Applications of Primordial and Mature Germ Cell Markers
Future research directions and applications of primordial and mature germ cell markers include:
| Applications | Details |
|---|---|
| Diagnostic and Prognostic Tools |
|
| Therapeutic Targeting |
|
| Reproductive Technologies |
|
| Regenerative Medicine |
|
| Cancer Research and Treatment |
|
| Genetic and Epigenetic Studies |
|
| Stem Cell Research and Development |
|
By further exploring and applying primordial and mature germ cell markers, researchers can unlock new diagnostic, therapeutic, and regenerative opportunities in reproductive medicine, cancer research, regenerative therapies, and beyond, ultimately advancing our understanding of germ cell biology and its implications for human health and reproduction.
Case Study
Case 1: Yang S, Ding S, He S, et al. Differentiation of primordial germ cells from premature ovarian insufficiency-derived induced pluripotent stem cells. Stem Cell Res Ther. 2019;10(1):156.
Premature ovarian insufficiency (POI) affects women's reproductive health, involving oocyte differentiation issues. Reprogramming fibroblasts from POI patients into induced pluripotent stem cells (iPSCs) allows for in-depth study of POI mechanisms and potential drug development.
By reprogramming fibroblasts from POI patients, including those with genetic conditions like fragile X syndrome, into iPSCs, researchers analyzed their characteristics and successfully induced differentiation into primordial germ cells (PGCs) using DNA methyltransferase inhibitors.
The study demonstrated the successful generation of disease-specific iPSC lines from POI patients and their efficient differentiation into PGCs, suggesting the potential of DNA demethylation to expedite this process. This research provides new cell models for studying POI pathogenesis and offers insights into potential treatments and egg resources for affected individuals.
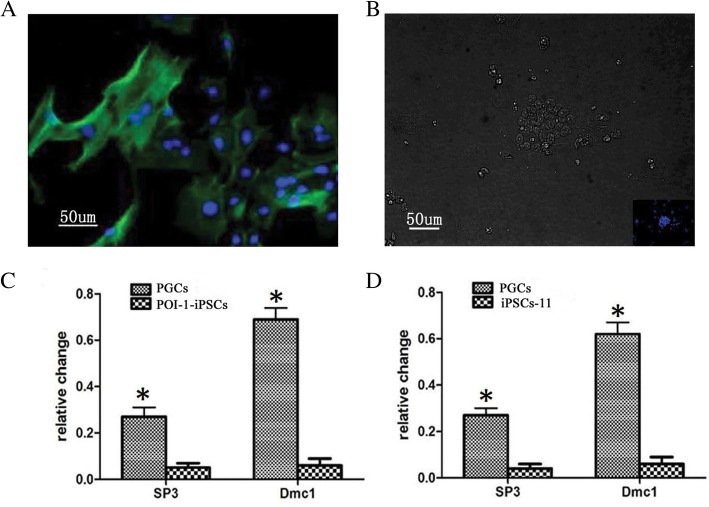 Fig.1 The induced PGCs from POI-1-iPSCs had the potential for meiotic progression.
Fig.1 The induced PGCs from POI-1-iPSCs had the potential for meiotic progression.Case 2: Luo YY, Jie HY, Huang KJ, et al. The dynamic expression of SOX17 in germ cells from the human female foetus and adult ovaries after specification. Front Endocrinol (Lausanne). 2023;14:1124143.
SOX17, crucial for human primordial germ cell specification, may play a role in germ cell development post-sex differentiation, a less understood area.
By examining gonadal samples from embryos, fetal ovaries, and adult ovaries, researchers labeled germ cells with SOX17, VASA, PHH3, and SCP3.
SOX17 was found in oogonia and oocytes during gestational weeks 15 to 28, shifting in location and expression levels over time. Co-expression with VASA ranged from 81.29% to 97.81% in foetal samples, with some SOX17+VASA- germ cells observed near the cortex, showing mitosis activity but not meiosis.
The study highlights the dynamic expression of SOX17 in female germ cells and the presence of a unique SOX17+ VASA- germ cell population in fetal ovaries. No clear link was established between SOX17 or VASA expression and mitosis/meiosis, suggesting SOX17's potential involvement in germ cell maturation post-specification, warranting further investigation.
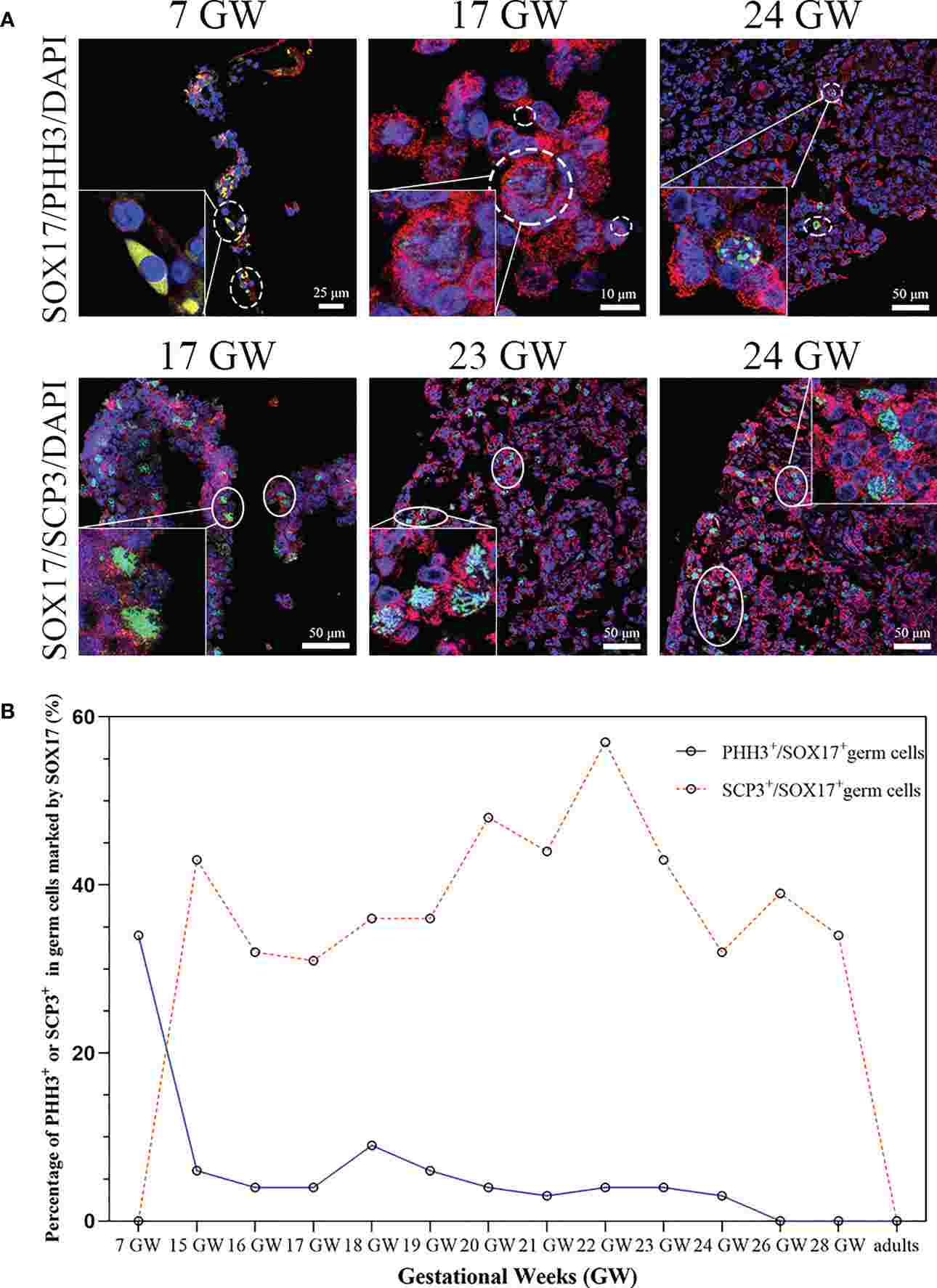 Fig.2 The percentage of mitosis or meiosis in SOX17 (red) immunopositive germ cells.
Fig.2 The percentage of mitosis or meiosis in SOX17 (red) immunopositive germ cells.Related References
- Hong T-K, Song J-H, Lee S-B, Do J-T. Germ cell derivation from pluripotent stem cells for understanding in vitro gametogenesis. Cells. 2021; 10(8):1889.
- Clark AT, Bodnar MS, Fox M, et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004;13(7):727-739.
- Alifi F, Asgari HR. Alteration in expression of primordial germ cell (PGC) markers during induction of human amniotic mesenchymal stem cells (hAMSCs). J Reprod Infertil. 2020;21(1):59-64.

