C Chemokines
Related Symbol Search List
Immunology Background
Available Resources for C Chemokines Research
Creative BioMart serves as your premier go-to destination for all your research requirements concerning C chemokines. Our vast array of meticulously crafted products and tailored services are meticulously curated to guide your exploration into the realm of C chemokines and their critical involvement in diverse physiological processes.
- Our product lineup features high-quality recombinant proteins, protein pre-coupled magnetic beads, cell and tissue lysates, and more.
- Furthermore, we provide a rich trove of resources on C chemokines, encompassing vital subjects like involved pathways, protein functions, interacting proteins, relevant articles, and areas of ongoing research.
Our Featured Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| XCL1-158H | Recombinant Human Lymphotactin, His-tagged | Human | E.coli | His |
| XCL2-593H | Recombinant Human XCL2 Protein, His-tagged | Human | E.coli | N-His |
About C Chemokines
C chemokines, also known as XC chemokines, are a subfamily of chemokines characterized by the arrangement of their conserved cysteine residues. Unlike other chemokine subfamilies, C chemokines have adjacent cysteine residues, with only one amino acid separating them.
C chemokines play important roles in immune responses, inflammation, and various physiological processes. They are produced by diverse cell types, including immune cells, endothelial cells, and stromal cells. These chemokines interact with specific receptors on immune cells, guiding their migration and activation.
Some notable C chemokines include:
- XCL1 (Lymphotactin): XCL1 is produced by activated T cells and natural killer (NK) cells. It acts as a chemoattractant for lymphocytes, particularly T cells and NK cells. XCL1 is involved in immune cell trafficking and the formation of lymphoid tissues.
- XCL2 (Lymphotactin-β): XCL2 is closely related to XCL1 and shares similar functions. It is also produced by activated T cells and NK cells and attracts lymphocytes to specific tissues.
C chemokines primarily participate in the recruitment and activation of lymphocytes, especially T cells and NK cells. They are involved in immune cell trafficking, immune surveillance, and the organization of lymphoid tissues. C chemokines contribute to the regulation of immune responses, aiding in the proper functioning of the immune system.
It's important to note that C chemokines constitute a small subfamily compared to other chemokine subfamilies such as CC and CXC. While their functions may be relatively less studied or characterized, they still play significant roles in immune cell biology and immune system regulation. Further research is needed to explore the full extent of their functions and potential therapeutic applications.
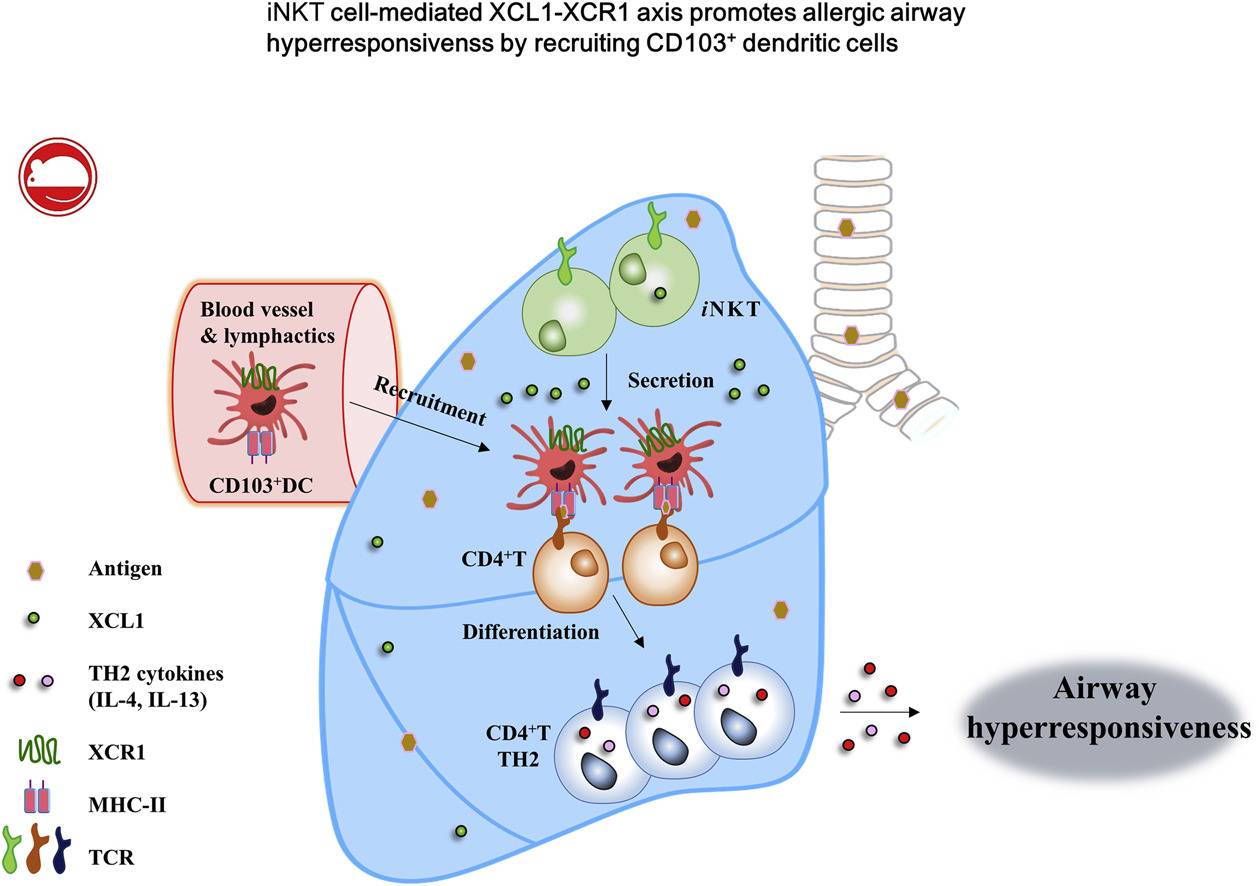 Fig.1 iNKT cell–mediated XCL1-XCR1 axis promotes AHR by recruiting CD103+ DCs into the lung in patients with allergic asthma. (Woo YD, et al., 2018)
Fig.1 iNKT cell–mediated XCL1-XCR1 axis promotes AHR by recruiting CD103+ DCs into the lung in patients with allergic asthma. (Woo YD, et al., 2018)
Research on C Chemokines
Research on C chemokines, particularly XCL1 and XCL2, has focused on understanding their roles in immune responses, inflammation, and various disease processes. Here are some key areas of research related to these C chemokines:
- Immune Cell Trafficking and Recruitment: XCL1 and XCL2 have been studied for their ability to attract specific subsets of immune cells, particularly T cells and NK cells. Research has investigated the mechanisms by which these chemokines mediate immune cell migration and their involvement in immune cell trafficking to specific tissues and organs. Understanding the role of XCL1 and XCL2 in immune cell recruitment contributes to our knowledge of immune surveillance, immune responses, and the development of immune-based therapies.
- Lymphoid Tissue Formation and Organization: XCL1 and XCL2 have been implicated in the formation and organization of lymphoid tissues, such as lymph nodes and Peyer's patches. These chemokines contribute to the positioning and compartmentalization of immune cells within lymphoid tissues, aiding in the generation of appropriate immune responses. Studies have explored the expression patterns of XCL1 and XCL2 in lymphoid tissues and their impact on lymphocyte localization and function.
- Inflammation and Autoimmunity: Investigations have examinedthe involvement of XCL1 and XCL2 in inflammatory processes and autoimmune diseases. These chemokines have been found to be upregulated in inflamed tissues and implicated in the recruitment and activation of immune cells during inflammation. Research has explored their roles in conditions such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. Understanding the contribution of XCL1 and XCL2 to inflammatory and autoimmune diseases may provide insights into potential therapeutic targets for modulating immune responses.
- Cancer Immunology: XCL1 and XCL2 have gained attention in the field of cancer immunology. Studies have investigated their roles in tumor immunity and the interactions between immune cells and cancer cells. XCL1 and XCL2 can influence the recruitment and activation of immune cells within the tumor microenvironment, potentially impacting tumor progression and antitumor immune responses. Research in this area aims to uncover the mechanisms underlying the immunomodulatory functions of XCL1 and XCL2 in cancer and explore their potential as therapeutic targets or biomarkers.
- Therapeutic Potential: The unique functions and expression patterns of XCL1 and XCL2 have sparked interest in their therapeutic potential. Manipulating these chemokines or their receptors may offer new strategies for modulating immune responses in various diseases. Researchers are exploring approaches such as targeting XCL1/XCL2 receptors to enhance or suppress immune cell recruitment, developing XCL1/XCL2-based therapies, or using these chemokines as biomarkers for disease diagnosis and prognosis.
It's important to note that research on C chemokines, including XCL1 and XCL2, is an active and evolving field. Ongoing studies continue to unravel their precise roles, mechanisms of action, and potential therapeutic applications in various immune-mediated disorders and malignancies.
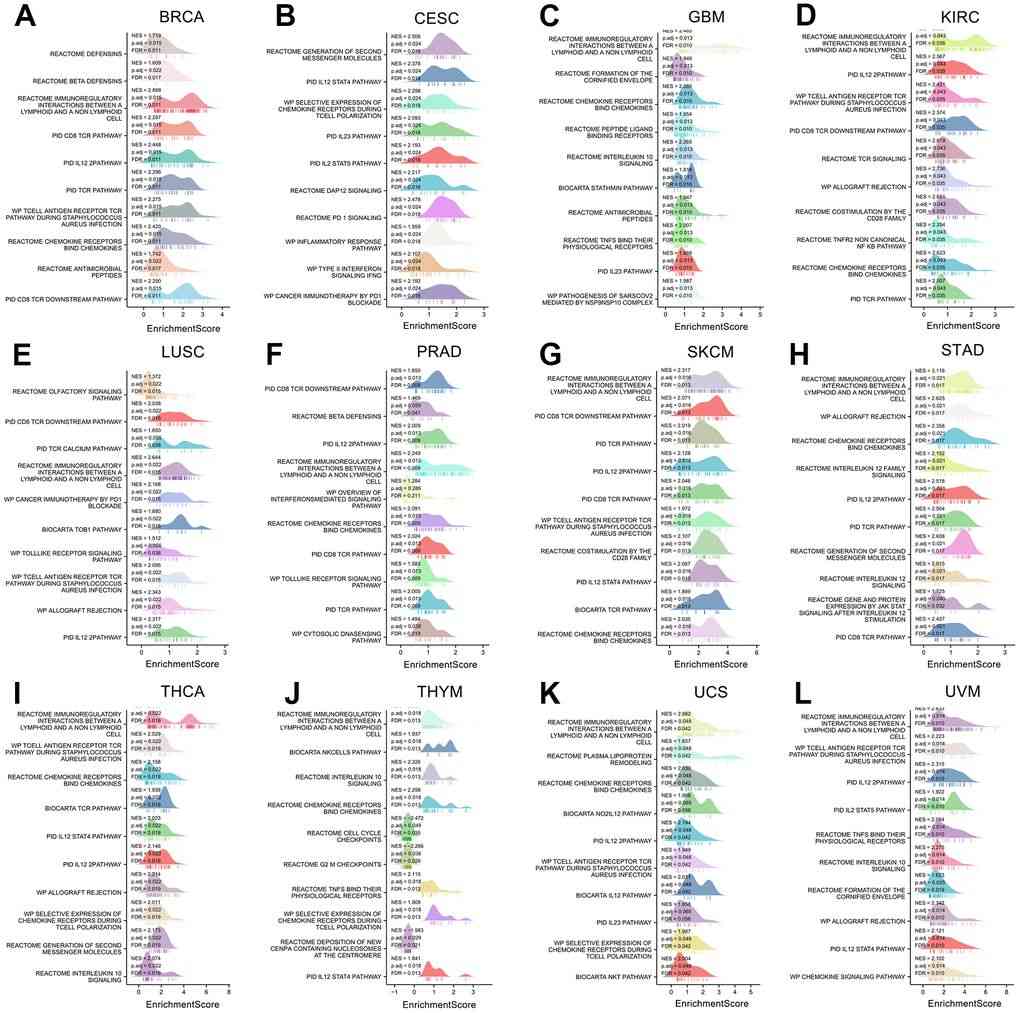 Fig.2 The gene set enrichment analysis of XCL2 in 12 cancers. The top 10 GSEA pathways of XCL2 in (A) BRCA, (B) CESC, (C) GBM, (D) KIRC, (E) LUSC, (F) PRAD, (G) SKCM, (H) STAD, (I) THCA, (J) THYM, (K) UCS, and (L) UVM. (Chen W, et al., 2023)
Fig.2 The gene set enrichment analysis of XCL2 in 12 cancers. The top 10 GSEA pathways of XCL2 in (A) BRCA, (B) CESC, (C) GBM, (D) KIRC, (E) LUSC, (F) PRAD, (G) SKCM, (H) STAD, (I) THCA, (J) THYM, (K) UCS, and (L) UVM. (Chen W, et al., 2023)
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
Related References
- Fox JC, Nakayama T, Tyler RC, Sander TL, Yoshie O, Volkman BF. Structural and agonist properties of XCL2, the other member of the C-chemokine subfamily. Cytokine. 2015;71(2):302-311.
- Chen W, Zou F, Song T, et al. Comprehensive analysis reveals XCL2 as a cancer prognosis and immune infiltration-related biomarker. Aging (Albany NY). 2023;15(21):11891-11917.
- Woo YD, Koh J, Kang HR, Kim HY, Chung DH. The invariant natural killer T cell-mediated chemokine X-C motif chemokine ligand 1-X-C motif chemokine receptor 1 axis promotes allergic airway hyperresponsiveness by recruiting CD103+ dendritic cells. J Allergy Clin Immunol. 2018;142(6):1781-1792.e12.

