Guide of Protein Subcellular Co-localization
Green fluorescence protein (GFP) has become one of the most interesting proteins in protein science research due to its unique physical and chemical properties. Because GFP has the ability to emit fluorescence in vivo, it can be used to detect and trace the expression level, location and protein interaction of GFP fusion protein. After the discovery of GFP fluorescent protein, people have carried out a series of mutation modifications on fluorescent protein, and obtained blue fluorescent protein (BFP), cyan fluorescent protein (CFP), yellow fluorescent protein (YFP), red fluorescent protein (RFP), etc., making the research of fluorescent protein more extensive.
In the research of protein-protein interaction, there are mainly protein subcellular co-localization, bimolecular fluorescence complementation (BiFC), fluorescence resonance energy transfer (FRET) and other methods using fluorescent protein. This section mainly introduces the preparation of Arabidopsis protoplasts and the detection of protein-protein interaction through the method of protein subcellular co-localization.
The purpose of this experiment manual is to help scientific researchers master the preparation of Arabidopsis protoplasts, learn the technology of detecting protein-protein interaction using protein subcellular co-localization method, and be familiar with the operation process and precautions of co-transfection technology.
Yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) are the most commonly used pair of fluorescent proteins to study the interaction between proteins and proteins in living cells. The excitation wavelength and absorption wavelength of YFP and CFP are different, and different wavelengths can be used for protein subcellular localization. The principle of protein subcellular co-localization to detect protein interaction is: when YFP-X or CEP-Y are transformed into cells alone, they are located in different subcellular compartments. However, when YFP-X and CFP-Y are transformed into a cell, they appear to be located in the same subcellular compartment, which indicates that X protein and Y protein may interact and thus be located together.
1. Main Instruments and Equipment
Micropipette (2 μL, 10 μL, 20 μL, 200 μL, 1000μL), desktop centrifuge, laser confocal microscope, thermostatic shaker, vacuum dryer, 75μm nylon membrane, slide, cover glass, 6-well tissue culture plate, 2mL centrifuge tube, 50mL centrifuge tube.
2. Material
pA7-CFP, pA7-YFP expression vector
3. Main Reagents
(1) Enzyme buffer
| MES (pH 5.7) | 20 mmol/L |
| Cellulase R10 | 1.5% |
| Macerozyme | 0.4% |
| Mannitol | 0.4mol/L |
| KCl | 20 mmol/L |
| Preheat the above solution at 55°C for 10min, cool at room temperature and add the following reagents | |
| CaCl2 | 10 mmol/L |
| BSA | 0.1% |
(2) WI solution
| MES (pH 5.7) | 4 mmol/L |
| Mannitol | 0.5 mol/L |
| KCl | 20 mmol/L |
(3) W5 solution
| MES (pH 5.7) | 2 mmol/L |
| NaCl | 154 mmol/L |
| KCl | 5 mmol/L |
| CaCl2 | 125 mmol/L |
(4) MMG solution
| MES (pH 5.7) | 4 mmol/L |
| Mannitol | 0.4 mol/L |
| MgCl2 | 15 mmol/L |
(5) PEG-calcium chloride transfection solution
| PEG 4000 | 30% |
| Mannitol | 0.4 mol/L |
| CaCl2 | 100 mmol/L |
1. Construction of Fusion Expression Vectors
(1) The target genes X and Y were respectively constructed into pA7-CFP or pA7-YFP expression vector *1.
(2) The constructed expression plasmid was sequenced and identified.
 Figure 4-4-1 Schematic Diagram of the Principle of Immunoprecipitation
Figure 4-4-1 Schematic Diagram of the Principle of Immunoprecipitation
2. Isolation of Protoplasts from Mesophyll Cells of Arabidopsis thaliana
(1) Select 3–4-week-old Arabidopsis seedlings with well extended leaves age *2 (Figure 4-4-1).
(2) Select 20-30 leaves and cut a 0.5-1.0 mm width from the middle part of the leaf with a sharp blade.
(3) Place the cut leaves quickly and gently into the prepared enzyme solution.
(4) Vacuum permeate the leaves with a desiccator in the dark for 30 min.
(5) Continue digestion without shaking for 3h*3 in the dark at room temperature.
(6) The protoplasts in the solution were examined microscopically and the size of Arabidopsis protoplasts was 30-50μm.
(7) Dilute the enzyme-protoplasm solution with the same volume of W5 solution.
(8) Remove undigested tissue by filtering protoplasts through a 75μm nylon membrane.
(9) Use a 50 mL tube, centrifuge at room temperature, 100g, for 2 min. to precipitate protoplasts.
(10) Remove the supernatant as much as possible and resuspend the protoplasm by gentle shaking.
(11) Under a microscope, after counting cells with a blood cell counter, resuspend protoplasts to 2×105/ mL with W5 solution.
(12) Allow to stand on ice for 30 min.
(13) Remove the W5 solution as much as possible without touching the protoplasts.
(14) Resuspend protoplasts to 2×105/ mL with MMG solution at room temperature.
3. PEG-calcium Chloride Mediated Cell Transfection
(1) Add 10μL of DNA to a 2 mL centrifuge tube*4.
(2) Add 100μL of protoplasts (2×104/ mL) and mix gently.
(3) Add 110μL PEG solution and mix gently.
(4) Incubate the transfection mixture at room temperature for 15 min.
(5) Dilute the transfection mixture with 400-440μL W5 solution at room temperature, and turn the centrifuge tube up and down to terminate the transfection.
(6) Centrifuge for 2 min at 100g at room temperature and remove the supernatant.
(7) Resuspend the protoplasts with 1 mL of WI solution and culture in 6-well tissue culture plates.
4. Protoplast Culture and Observation
(1) Incubate the protoplasts for 16h at room temperature.
(2) Centrifuge the protoplasts at room temperature and 100g for 2 min and collect the protoplasts.
(3) The protoplasts were placed on a slide, covered with a coverslip, and observed under a laser confocal microscope*5 (Figure 4-4-2).
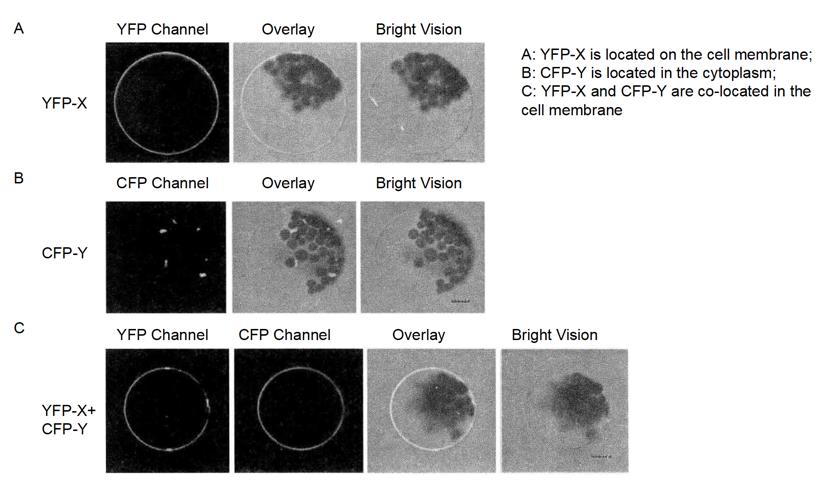 Figure 4-4-2 Observation of Protein Interaction by Subcellular Co-localization
Figure 4-4-2 Observation of Protein Interaction by Subcellular Co-localization
1. The premise of using protein subcellular co-localization is that two proteins are located in different subcellular compartments and transferred to one cell in total, and one protein changes in location and is located with the other protein.
2. The experiment of protein subcellular co-localization needs to be transferred into cells, and protoplasts or cell lines can be prepared according to the needs of the experiment, such as BY2 cells of tobacco.
*1 The pA7-CFP or pA7-YFP expression vector is driven by the 35S promoter to express the fusion protein in plant cells.
*2 Arabidopsis was incubated with short daylight and controlled temperature and humidity.
*3 When the leaves are well enzymatically digested, the solution should be green.
*4 5-10 kb of plasmid DNA, 10-20 g were added.
*5 The localization of CFP and YFP fusion proteins was detected separately by different channels under laser confocal microscope.

