Avi-tagged Proteins
🧪 CD40LG-280H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: 113-261 a.a.
🧪 FCGR1A-3817H
Source: HEK293
Species: Human
Tag: Avi&His
Conjugation: Biotin
Protein Length: Met 1-Thr 287
🧪 Fcgr4-3997M
Source: HEK293
Species: Mouse
Tag: Avi&His
Conjugation: Biotin
Protein Length: Met 1-Gln 203
🧪 CTLA4-256H
Source: HEK293
Species: Human
Tag: Avi&mFc
Conjugation: Biotin
Protein Length: 37-162 a.a.

🧪 CD226-636H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: Glu 19 - Asn 247
🧪 PVR-653H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: Trp 21 - Asn 343
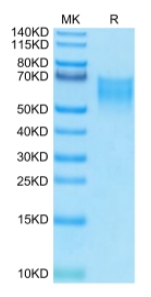
🧪 CD86-581H
Source: HEK293
Species: Human
Tag: Avi&His
Conjugation: Biotin
Protein Length: Leu26-Pro247
🧪 CD28-3908H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: Asn 19 - Pro 152
🧪 HA-412I
Source: HEK293
Species: H9N2
Tag: Avi&His
Conjugation: Biotin
Protein Length: 19-338 a.a.
🧪 CD27-57H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: Thr 21 - Ile 192
🧪 TNFRSF17-238H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: Met 1 - Ala 54
🧪 CD40-2222H
Source: HEK293
Species: Human
Tag: Avi&His
Conjugation: Biotin
Protein Length: Glu21-Arg193
🧪 CD86-585H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: Leu 26 - Pro 247
🧪 CD244-1825H
Source: HEK293
Species: Human
Tag: Avi&Fc
Conjugation: Biotin
Protein Length: AA Cys 22 - Arg 221
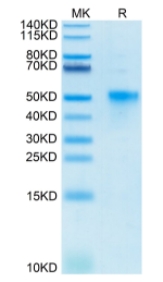
🧪 TNFRSF4-1826H
Source: HEK293
Species: Human
Tag: Avi&His
Conjugation: Biotin
Protein Length: Leu29-Ala216
Background
Overview
An avi-tagged protein is a protein with an Avi tag added through genetic engineering. The Avi tag is a 15 amino acid peptide tag with the sequence Gly-Leu-Asn-Asp-Ile-Phe-Glu-Ala-Gln-Lys-Ile-Glu-Trp-His-Glu. It is a popular fusion tag with powerful and versatile properties. Biotinylation of the Avi tag is to covalently attach a biotin molecule to the lysine residue within the Avi-tag with E. coli biotin ligase (BirA). In vitro, BirA enzyme-labeled biotin efficiency is over 95%. This binding enables the Avi-tagged protein to be biotinized in the cell or in vitro, i.e. covalently linked to biotin (a vitamin H).
The development of Avi protein labeling technology provides an efficient, consistent labeling method with little influence on protein activity for protein research, which is of great significance for monitoring biological processes, auxiliary detection and protein purification.
Strategies
Introduction of Avi tag : An Avi tag consisting of 15 amino acids is introduced at the N or C terminal of the target protein. This tag can be recognized by E. coli biotin ligase BirA.
Biotinization : Biotin is site-attached to a specific lysine residue in the Avi tag using the enzyme BirA. This process occurs in vitro or in vivo and is extremely efficient, with over 95% labeling efficiency.
Maintenance of activity : Since biotinization occurs at the end of the protein, rather than the active center of the protein, the structure and function of the protein are maintained without affecting its biological activity.
Uniform orientation : When Avi labeled proteins bind to streptavidin coated surfaces, there is a high degree of consistency in protein orientation due to the precise control of biotinization. This helps to improve the efficiency and repeatability of the experiment.
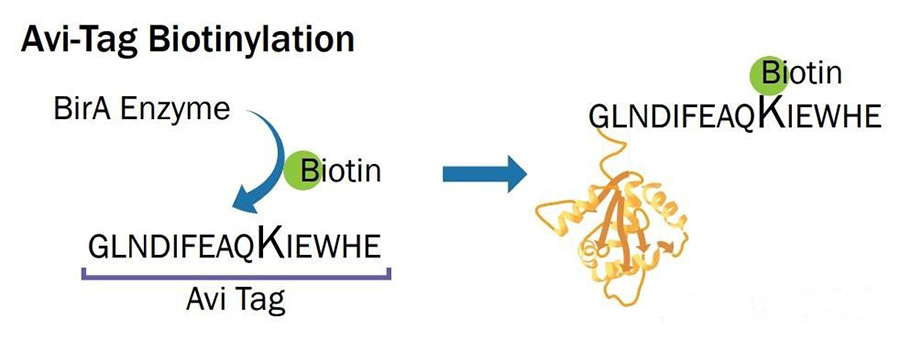
Fig1. Schematic diagram of biotin ligase at work.
Advantages
This type of protein has the following properties:
Consistency of labeling : Avi labeling technology enables site-specific biotinization at specific locations in the protein, and this labeling does not affect the activity of the protein because it occurs on a single lysine residue labeled by Avi.
Unity of protein orientation : When Avi labeled proteins bind to streptavidin coated surfaces, the protein orientation is highly consistent due to the precise control of biotinization. This is particularly important to improve the efficiency of the combined analysis experiment.
Wide applicability : The Avi Tag system can be used to label almost all proteins, both in vitro and in vivo, and can be easily and efficiently biotinized at a unique Avi TAG site.
Maintenance of high biological activity : Avi labeled protein has been verified by ELISA, SPR, BLI and other methods, showing high biological activity, which means that the labeled protein can maintain its original biological function.
High batch consistency : Avi labeling technology provides high batch consistency of labeled proteins, which is critical for repeatability and accuracy of experiments.
Advantages of short peptide tag : Avi-tag is a short peptide tag composed of 15 amino acid residues, which allows it to be efficiently recognized and biotinized by biotin ligase (BirA) in vivo or in vitro.
Applications
Fused to the protein, the Avi tag provides a multi-functional system useful for many applications, including: expression, imaging, detection, localization, isolation, immobilization. In addition, Avi-tag system protein can be simply and efficiently purified or adsorption fixed. Its number of applications in medical and industrial fields continues to increase.
- Experimental studies: Avi-tagged protein can be used in various biomedical experiments, such as enzyme-linked immunosorbent assay (ELISA), flow cytometry (FACS), Biopanning.
- Clinical studies: Avi-tagged protein can be used to tag and track cells, and can also be used to analyze the immune response caused by vaccines and assist in screening.
- Drug development: The Avi-tagged protein can be used as a target or detection tool to help researchers evaluate the effectiveness and specificity of antibodies. Or as a diagnostic tool for detecting and diagnosing various diseases.
Case Study
Case Study 1: Recombinant Human CCL2 protein, His-Avi-tagged, Biotinylated
Clinical trials have demonstrated reductions in major adverse cardiovascular events with purified high-dose eicosapentaenoic acid (EPA), independent of effects on lipids. The researchers aimed to investigate whether omega-3 fatty acids reduce vascular inflammation, a critical mediator of atherosclerosis, and hypothesised that EPA is superior to docosahexaenoic acid (DHA).
In a double-blind randomised controlled trial and cell-culture study, 40 healthy volunteers were supplemented with 4 g daily of either EPA, DHA, fish oil (2:1 EPA:DHA), or placebo for 30 days. Serum was incubated with TNF-stimulated human umbilical vein endothelial cells (HUVECs), and markers of acute vascular inflammation (AVI) were measured. EPA supplementation reduced expression of C-C motif chemokine ligand 2 (CCL2) by 25% compared to placebo (p = 0.03). In the AVI model, EPA reduced vascular expression of VCAM1 by 43% (p = 0.02) and CCL2 by 41% (p = 0.03). Significant inverse correlations were observed between EPA levels and vascular expression of VCAM1 (r = -0.56, p = 0.001) and CCL2 (r = -0.56, p = 0.001).
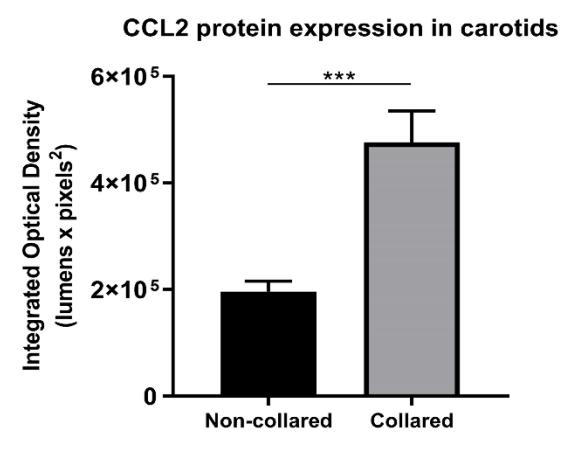
(Anthony D. Pisaniello, 2021)
Fig1. Carotid collaring induced markers of acute vascular inflammation. Collared and non-collared arteries underwent immunohistochemical staining for CCL2.
Case Study 2: Recombinant Human EGFR protein, His&Avi-tagged, Biotinylated
Hybridoma technology is widely used for monoclonal antibody (mAb) discovery, whereas the generation and identification of single hybridomas by the limiting dilution method (LDM) are tedious, inefficient, and time- and cost-consuming, especially for hapten molecules. Here, the researchers describe a single transgenic hybridoma selection method (STHSM) that employs a transgenic Sp2/0 with an artificial and stable on-cell-surface anchor. The anchor was designed by combining the truncated variant transmembrane domain of EGFR with a biotin acceptor peptide AVI-tag, which was stably integrated into the genome of Sp2/0 via a piggyBac transposon.
To ensure the subsequent precise selection of the hybridoma, the number of on-cell-surface anchors of the transfected Sp2/0 for fusion with immunized splenocytes was further normalized by flow cytometry at the single cell level. The best affinity of mAbs from the STHSM was more than 2-fold higher than that of those from the LDM, and this was mainly due to the preaffinity selection based on the on-cell-surface anchors and more interactions between the mAb and CAP. Then the mAbs from the STHSM and LDM were used to develop an immunoassay for CAP in spiked and natural biological samples.

(Yuan Li, 2022)
Fig2. The AVI-tag-EGFR on-cell-surface anchor, was constructed. Western blot analysis of the AVI-tag protein expression with the anti-AVI-tag antibody. Top: protein expression of AVI-tag. Bottom: protein expression of GAPDH as a control.
Case Study 3: Recombinant Human EGFR protein, His-Avi-tagged, Biotinylated
The vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) pathway is considered to be one of the most important regulators of angiogenesis and a key target in anticancer treatment. Imaging VEGFR expression can serve as a new paradigm for assessing the efficacy of antiangiogenic cancer therapy, improving cancer management, and elucidating the role and modulation of VEGF/VEGFR signaling during cancer development and intervention. In this study we developed an Avi-tagged VEGF(121) protein, which is site-specifically biotinylated in the presence of bacterial BirA biotin ligase. BirA biotinylated VEGF(121)-Avi (VEGF(121)-Avib) forms a stable complex with streptavidin-IRDye800 (SA800) that retains high affinity for VEGFR in vitro and allows receptor specific targeting in vivo in a 67NR murine xenograft model. In contrast, chemical coupling of IRDye800 abrogated the VEGFR binding ability of the modified protein both in vitro and in vivo.
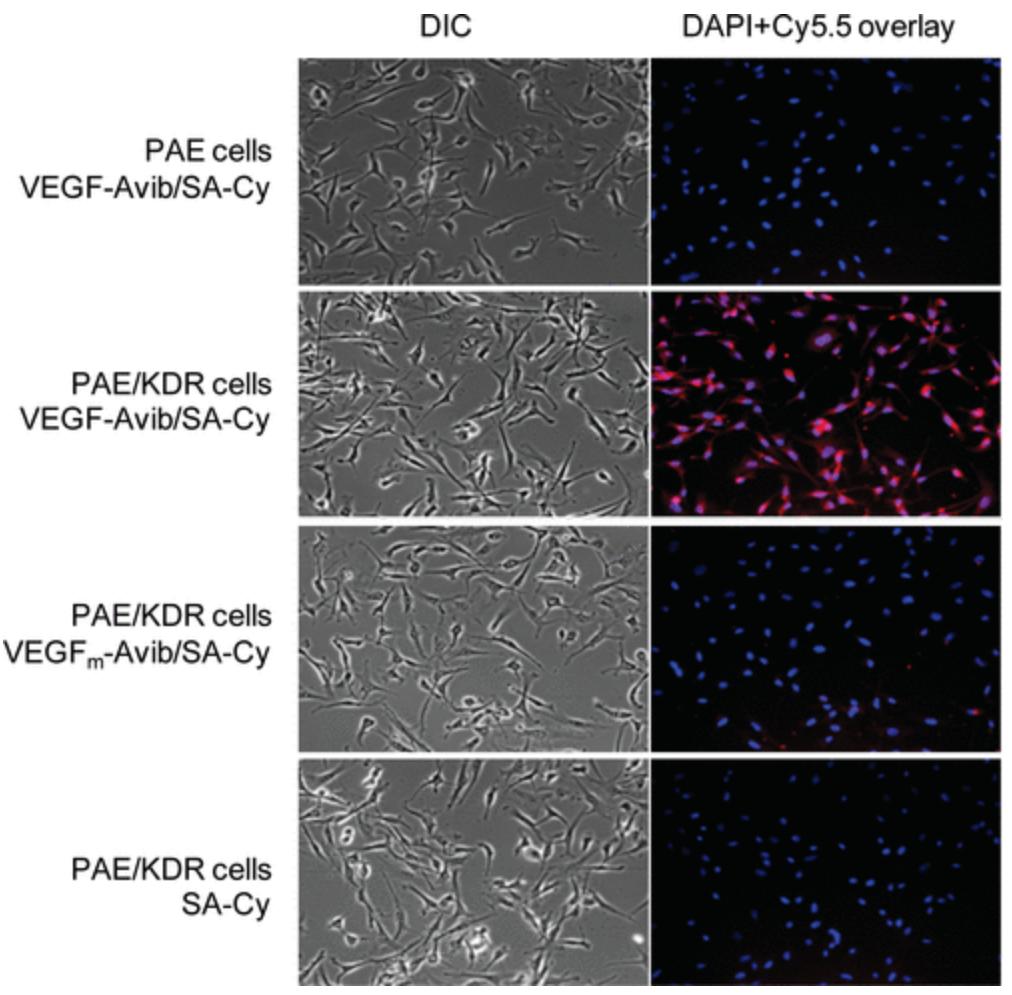
(Hui Wang, 2009)
Fig3. Cell staining of Cy5.5 conjugates. Differential interference contrast (DIC) and epifluorescence images of PAE cells with VEGF-Avib/SA-Cy and PAE/KDR cells with VEGF-Avib/SA-Cy, VEGFm-Avib/SA-Cy and SA-Cy.
Case Study 4: Recombinant Alpaca VHH protein, His-Avi-tagged, Biotinylated
A simple and sensitive fluoroimmunoassay (FIA) based on a heavy-chain antibody (VHH) for rapid detection of fenitrothion was developed. A VHH library was constructed from an immunized alpaca, and one clone recognizing fenitrothion (namely, VHHjd8) was achieved after careful biopanning. It was biotinylated by fusing with the Avi tag and biotin ligase to obtain a fusion protein (VHHjd8-BT), showing both binding capacity to fenitrothion and the streptavidin poly-horseradish peroxidase conjugate (SA-polyHRP). Based on a competitive assay format, the absorbance spectrum of oxidized 3,3',5,5'-tetramethylbenzidine generated by SA-polyHRP overlapped the emission spectrum of carbon dots, which resulted in quenching of signals due to the inner-filter effect. The developed FIA showed an IC50 value of 1.4 ng/mL and a limit of detection of 0.03 ng/mL, which exhibited 15-fold improvement compared with conventional enzyme-linked immunosorbent assay.
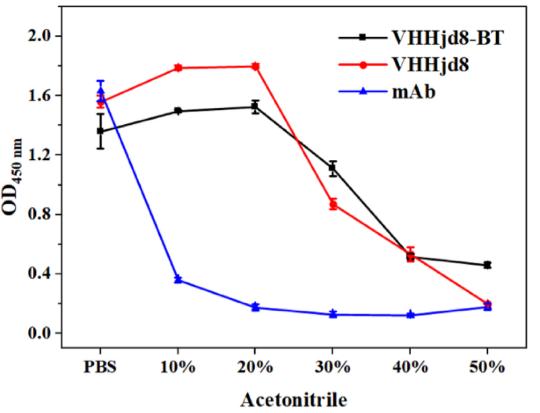
(Zi-Jian Chen, 2022)
Fig4. Stability study of anti-fenitrothion VHHjd8 and the corresponding mAb. Effect of acetonitrile on binding activity to hapten 2-BSA.
Advantages
- Wide Coverage : More than 300 Avi-tagged proteins from over 10 different species and 4 different sources.
- High Quality : The Avi-tag biotinylated recombinant proteins ensure consistent labeling, uniform protein orientation and equivalent bioactivity. And they have been tested with different methods to ensure intergrity and high purity.
- Experienced : We have professional research team with experience of many years in the field of molecular and cell biology.
- Fast turnaround : Under the premise of your protein expression and purification, as little as 4-6 weeks.
FAQ
-
Q: What are the advantages of enzyme-catalyzed biotin labeling over chemically labeled biotin?
A: Almost all proteins can be easily and efficiently biotinized at a unique Avi-tag site, either in vitro or in vivo. Since such biotinylation is achieved through the reaction of enzymes and substrates, the reaction conditions are quite mild and the specificity of the labeling is extremely high. The biotin label of the chemical method is 85 amino acids, while the biotin Avi-tag has only 15 amino acids and is one-fifth the size of the former.
-
Q: What are the expression systems for Avi-tagged proteins?
A: We have a variety of protein expression synergies, including prokaryotic protein expression system, insect cell protein expression system and mammalian cell protein expression system.
Related Resource
- A Comprehensive Comparison of Avi-tag with Other Biotinylation Tags
- The Science behind Avi-tag: Understanding Biotinylation Mechanisms
- Step-by-Step Protocol for In Vitro Biotinylation of Avi-tagged Proteins
- Applications of Avi-tag Technology in Industry
- The Versatile Applications and Benefits of Avi-tag in Protein Research
References
- Wang, H.; et al. Site-specifically biotinylated VEGF(121) for near-infrared fluorescence imaging of tumor angiogenesis. Mol Pharm . 2009;6(1):285-294.
- Li, Y.; et al. Monoclonal Antibody Discovery Based on Precise Selection of Single Transgenic Hybridomas with an On-Cell-Surface and Antigen-Specific Anchor [published correction appears in ACS Appl Mater Interfaces. 2023 Sep 20;15(37):43223-43225]. ACS Appl Mater Interfaces. 2022;14(15):17128-17141.
- Pisaniello, AD.; et al . Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Atherosclerosis . 2021;324:27-37.
- Chen ZJ.; et al. Production and Characterization of Biotinylated Anti-fenitrothion Nanobodies and Development of Sensitive Fluoroimmunoassay. J Agric Food Chem. 2022;70(13):4102-4111.


