H1 Family
Related Symbol Search List
Immunology Background
Background
Histone H1 is a family of proteins that plays a crucial role in chromatin structure and function. The Histone H1 family comprises multiple variants, including H1.1, H1.2, H1.3, H1.4, H1.5, H1.0 (H1x), and others. These variants are encoded by separate genes and are expressed in a tissue-specific and developmentally regulated manner.
Structure
Histone H1 proteins are relatively small in size and are characterized by a tripartite structure. They consist of a central globular domain flanked by short N-terminal and C-terminal tails. The globular domain is involved in binding to DNA, while the tails are modified by various post-translational modifications, such as phosphorylation, acetylation, and methylation.
Function and Role in Biological Processes
- Chromatin compaction: Histone H1 proteins bind to the linker DNA between nucleosomes and facilitate the folding of chromatin into higher-order structures. They promote the compaction of nucleosomal arrays, contributing to the formation of higher-order chromatin fibers.
- Regulation of gene expression: Histone H1 proteins can influence gene expression by modulating chromatin accessibility. They can stabilize or destabilize nucleosome positioning, thereby facilitating or inhibiting the binding of transcription factors and other regulatory proteins to DNA. Histone H1 variants may exhibit differential effects on gene expression, contributing to the regulation of specific sets of genes.
- Transcriptional regulation: Histone H1 proteins are involved in the regulation of transcriptional elongation and pausing. They interact with RNA polymerase II and transcription elongation factors, influencing the rate of transcription elongation and the release of paused polymerases.
- Cell division: Histone H1 proteins play a role in mitotic chromosome condensation and segregation. During mitosis, they undergo phosphorylation and dissociate from chromosomes, allowing for chromosome condensation. Upon completion of mitosis, they are reassembled onto the chromatin.
- DNA repair and recombination: Histone H1 proteins participate in the repair of DNA damage and the regulation of DNA recombination processes. They can influence the accessibility of damaged DNA to repair enzymes and modulate the efficiency and fidelity of DNA repair mechanisms.
- Epigenetic regulation: Histone H1 proteins are involved in the establishment and maintenance of epigenetic marks. They can interact with DNA methyltransferases and histone-modifying enzymes, contributing to the establishment and propagation of specific histone and DNA modifications associated with gene silencing or activation.
Overall, the Histone H1 family plays a critical role in chromatin structure, gene expression regulation, cell division, and various other biological processes. The different variants of Histone H1 may have distinct functions and contribute to the fine-tuning of gene expression and chromatin dynamics in different cellular contexts.
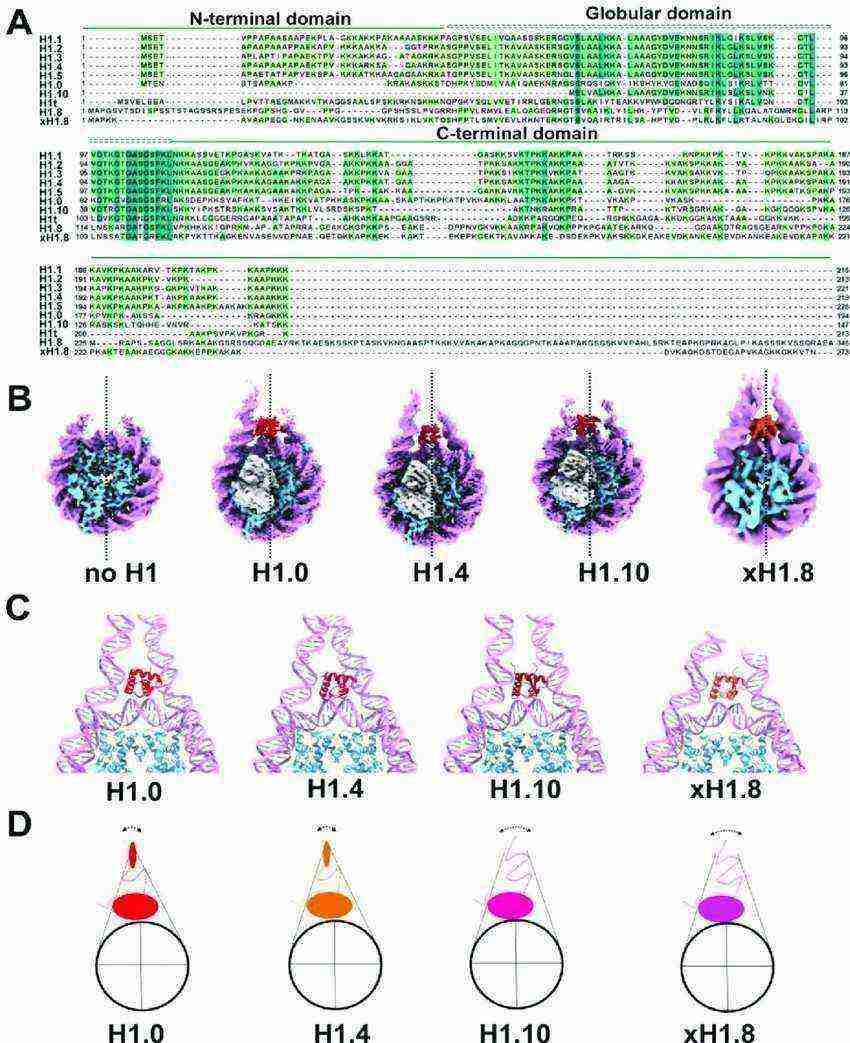 Fig.1 Structural diversity of linker histone H1 and chromatosome. (Sokolova V, et al., 2022)
Fig.1 Structural diversity of linker histone H1 and chromatosome. (Sokolova V, et al., 2022)Members and Variants of the H1 Family
| Members and variants | Details |
|---|---|
| H1F0 | H1F0, also known as H1.0 or H1°, is a replication-independent variant of histone H1. It is expressed in a wide range of tissues and is involved in chromatin compaction and gene regulation. |
| H1FNT | H1FNT is a testis-specific variant of histone H1. It is expressed specifically in the testis and plays a role in chromatin condensation during spermatogenesis. |
| H1FX | H1FX, also known as H1.10, is a variant of histone H1 that is found on the X chromosome. It is involved in chromatin compaction and gene regulation, similar to other H1 variants. |
| HIST1H1A | HIST1H1A corresponds to the H1.1 variant of histone H1. It is one of the major variants and is involved in chromatin compaction and gene regulation. |
| HIST1H1B | HIST1H1B corresponds to the H1.2 variant of histone H1. It is another major variant and plays a role in chromatin structure and gene expression regulation. |
| HIST1H1C | HIST1H1C corresponds to the H1.3 variant of histone H1. It is primarily expressed in embryonic stem cells and is involved in the maintenance of pluripotency and self-renewal. |
| HIST1H1D | HIST1H1D corresponds to the H1.4 variant of histone H1. It is highly expressed in testis and plays a role in chromatin compaction during sperm development. |
| HIST1H1E | HIST1H1E corresponds to the H1.5 variant of histone H1. It is expressed in a tissue-specific manner, with high levels found in brain tissue. It has been implicated in neuronal differentiation and function. |
| HIST1H1T | HIST1H1T corresponds to the H1.10 variant of histone H1, which is the same as H1FX. It is found on the X chromosome and is involved in chromatin compaction and gene regulation. |
Role of Histone H1 Family in Disease Development and Research Advances
The role of the histone H1 family in disease development and research progress is a complex and evolving field. Here are some examples highlighting their involvement in specific diseases and recent research advances:
Cancer
- Histone H1 variants have been associated with cancer development and progression. For example, altered expression of H1 variants has been observed in various cancers, including breast, lung, and prostate cancer.
- Research has shown that changes in the post-translational modifications of histone H1, such as phosphorylation and acetylation, can impact gene expression patterns and contribute to tumor formation.
- Recent studies have explored the potential of targeting histone H1 modifications as a therapeutic approach in cancer treatment. For instance, inhibiting specific histone H1 modifications may help restore normal gene expression patterns and inhibit tumor growth.
Neurodegenerative diseases
- Dysregulation of histone H1 and other histones has been implicated in neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, and Huntington's disease.
- Research has shown that alterations in histone H1 modifications can affect gene expression in neurons, leading to neuronal dysfunction and neurodegeneration.
- Recent studies have focused on understanding the epigenetic changes associated with neurodegenerative diseases and exploring the potential of targeting histone H1 modifications for therapeutic interventions.
Epigenetic research
- Histone H1 variants and their modifications are a subject of intensive epigenetic research. Advances in genomic and proteomic technologies have enabled the characterization of histone H1 variants, their post-translational modifications, and their interactions with other chromatin components.
- Researchers have identified specific histone H1 modifications that are associated with gene activation or repression, shedding light on the mechanisms of gene regulation and chromatin organization.
- Recent studies have focused on elucidating the roles of histone H1 variants in different cellular processes, including DNA repair, replication, and transcriptional regulation, to gain a deeper understanding of their contributions to normal and disease states.
Biomarkers and diagnostics
- Histone H1 modifications hold promise as biomarkers for disease diagnosis and prognosis. Epigenetic alterations in histone H1 have been associated with various diseases, and their detection in patient samples may provide valuable information for disease stratification and treatment decisions.
- Research efforts have focused on identifying specific histone H1 modifications or epigenetic signatures associated with diseases, such as cancer subtypes or neurological disorders, to develop diagnostic tools and improve patient management.
These examples highlight the diverse roles of the histone H1 family in disease development and the progress made in understanding their involvement through research. However, it's important to note that this field is still evolving, and further studies are needed to fully elucidate the intricate mechanisms and potential therapeutic applications of histone H1 in various diseases.
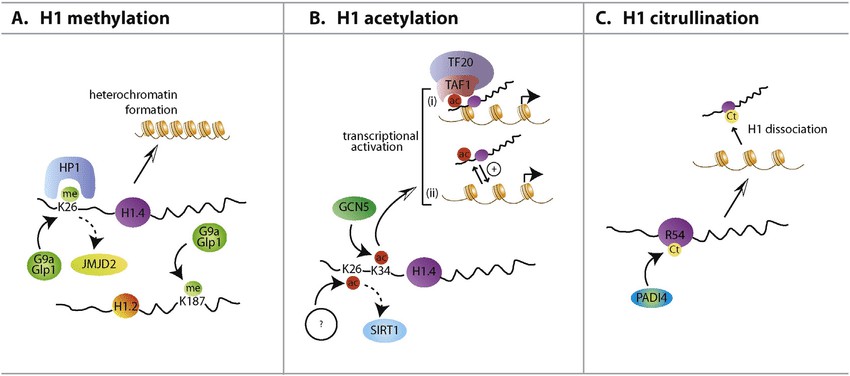 Fig.2 H1 modifications and their function. (Izzo A, et al., 2016)
Fig.2 H1 modifications and their function. (Izzo A, et al., 2016)Case Study
Case 1: Hu S, Chapski DJ, Gehred ND, et al. Histone H1.0 couples cellular mechanical behaviors to chromatin structure. Nat Cardiovasc Res. 2024;3(4):441-459.
The study investigated the role of histone H1.0 in controlling responses to physical stress in vivo. The authors used a model of catecholamine stimulation with ISO, a β-adrenergic receptor agonist that increases cardiac work and induces fibrosis. They found that administration of ISO led to cardiac muscle hypertrophy and impaired diastolic function, which were attenuated by siRNA-mediated depletion of histone H1.0. Depletion of histone H1.0 also blocked the activation of fibrotic genes and prevented ISO-induced fibrosis. Additionally, depletion of histone H1.0 using an alternative technique yielded similar results. These findings demonstrate that histone H1.0 plays a crucial role in regulating fibrosis in vivo through its actions on chromatin packaging.
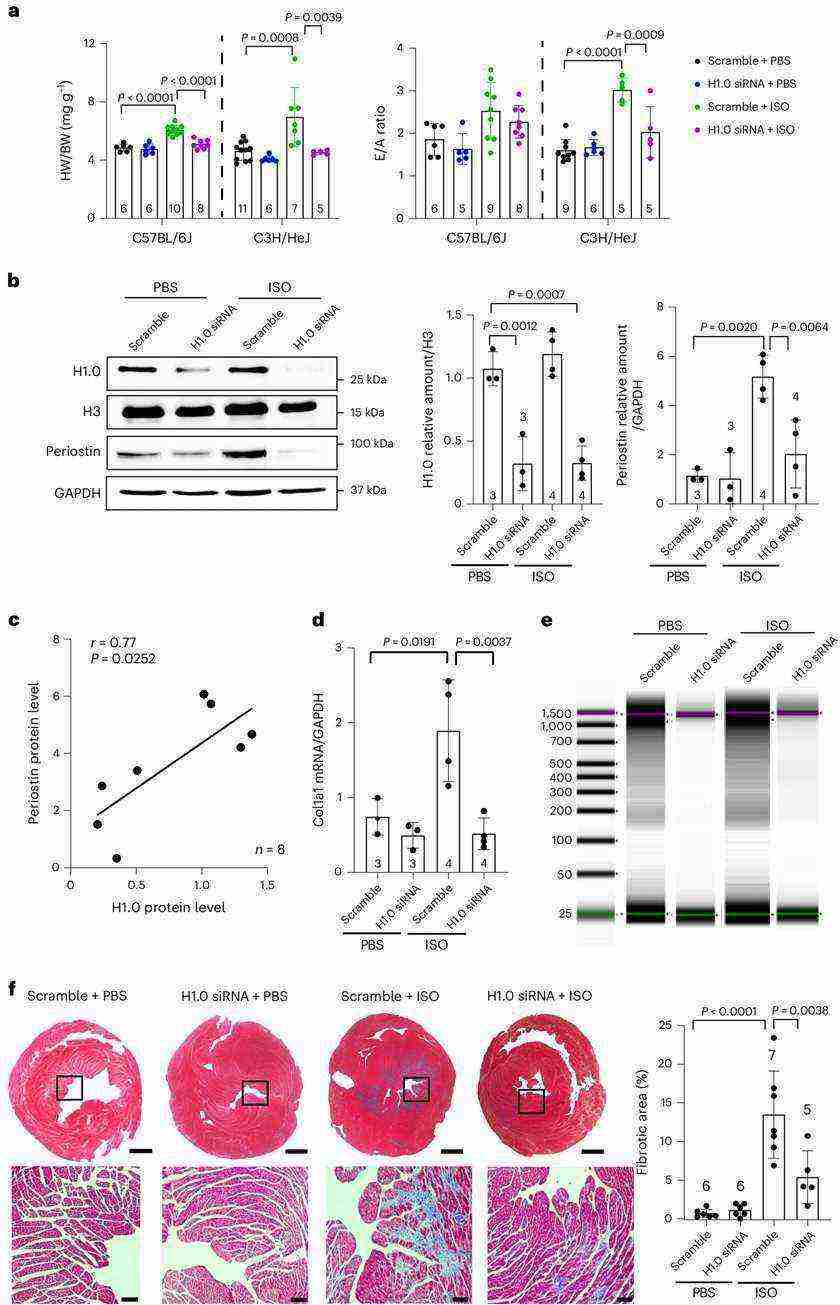 Fig.1 Histone H1.0 depletion prevents disease-associated cardiac fibrosis in vivo.
Fig.1 Histone H1.0 depletion prevents disease-associated cardiac fibrosis in vivo.Case 2: Sancho M, Diani E, Beato M, Jordan A. Depletion of human histone H1 variants uncovers specific roles in gene expression and cell growth. PLoS Genet. 2008;4(10):e1000227.
The study focused on the role of histone H1 variants in gene regulation and cell growth in a human breast cancer cell line. Using inducible shRNA-mediated knockdown, the researchers investigated the specific functions of each H1 variant. They found that depletion of H1.2 and H1.4 led to slower cell growth and failure to reach confluency. H1.4 knockdown resulted in increased mortality, possibly through necrosis, while H1.2 knockdown caused cell cycle arrest in the G1 phase. The effects of H1.2 depletion were observed even in the presence of damaging agents that block cell cycle progression. These findings indicate that histone H1 variants have distinct roles in regulating gene expression and cell growth, with H1.4 being essential for cell survival and H1.2 involved in G1 arrest.
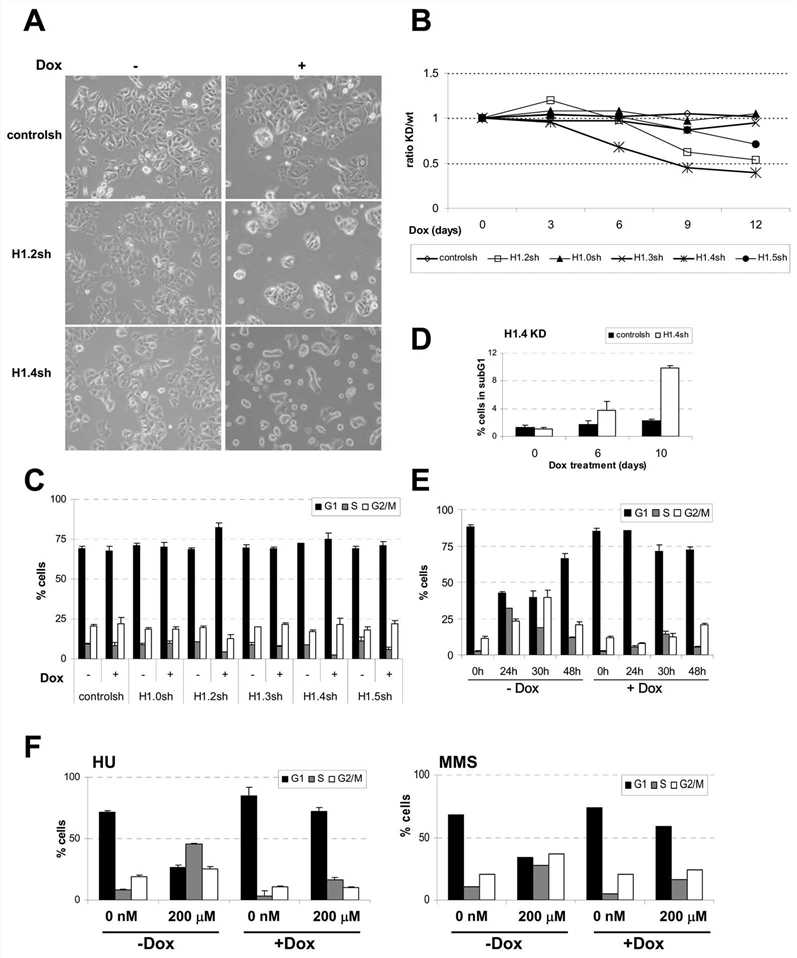 Fig.2 Inhibition of H1 variants causes different effects on cell proliferation and cell cycle progression.
Fig.2 Inhibition of H1 variants causes different effects on cell proliferation and cell cycle progression.References
- Sokolova V, Sarkar S, Tan D. Histone variants and chromatin structure, update of advances. Comput Struct Biotechnol J. 2022;21:299-311.
- Izzo A, Schneider R. The role of linker histone H1 modifications in the regulation of gene expression and chromatin dynamics. Biochim Biophys Acta. 2016;1859(3):486-495.

