Guide of Analysis of Promoter Regulatory Elements Based on Plant CARE Prediction Method
PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) is a database of plant cis-acting elements, enhancers and inhibitors. The database provides information about the location of regulatory elements, consistent sequences and independent sites on specific promoter sequences. At the same time, links to EMBL, TRANSFAC and MEDLINE databases are also provided. The data of the transfer sites in the database are mainly from the existing literature reports, plus some data predicted by the network. In addition to the general description of the specific transcription factor binding sites, the confidence of the experimental evidence, the information related to the function and the position on the promoter are also described. In the query sequence, plant cis-acting elements can be queried according to the known sequence information. In addition, a new clustering and motif search method is also provided to study the connection of co-expression gene sets. New control elements can also be automatically sent and added to the database after maintenance. Plant-CARE related databases can be accessed through the World Wide Web (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
PlantCARE database software for plant cis-acting element analysis (http://bioinformatics.psb.ugent.be/webtools /plantcare/html/).
1. Main Instruments and Equipment
A computer with a web browser that can be connected to the Internet.
2. Material
The characteristic promoter sequence of the target gene.
Enter the PlantCARE database web address in the browser (http://bioinformatics.psb.ugent.be/webtools /plantcare/html/), enter the homepage. There are two main button menus, Query CARE and Search for CARE. Query CARE is mainly used to retrieve relevant information of specific elements, while Search for CARE is mainly used to retrieve relevant cis-acting elements of specific promoter sequences.
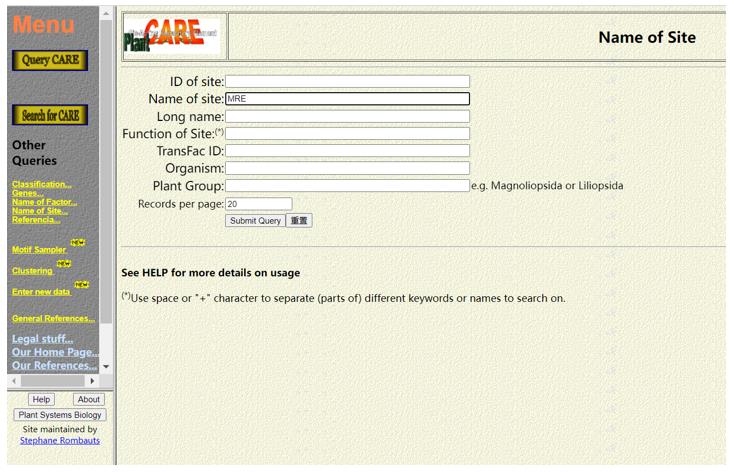
Information about cis-acting elements can be searched by clicking Query CARE and entering relevant information. For example, if we want to retrieve information about "components" of MYB reaction, we can enter MRE in the Name of the Site to enter the search page. Of course, you can also enter other information, such as Gene ID, tissue, cell type, function or sequence.
The search results about MRE will appear on the search results page. It includes simple functional description, tissue source, gene ID number and element sequence.

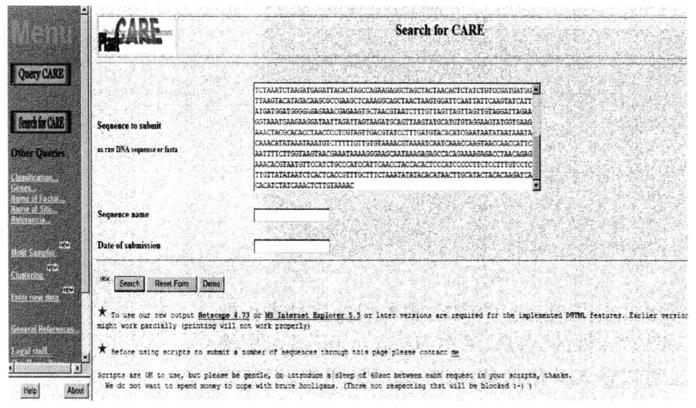
If you want to search for the same or similar cis-acting elements in the known sequence as reported in the PlnatCARE database, you can use Search for CARE. Paste the nucleotide sequence to be analyzed into the query box (note that the length of the submitted sequence should not exceed 1500 bp), and click Search. The web page will return the analysis results of the submitted sequence.
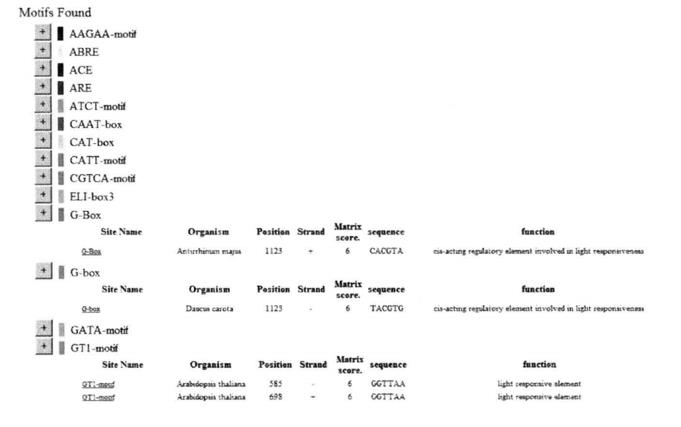
The results include the site name, tissue source, position in the sequence, positive and negative chain, matching score, sequence and function and other relevant information.
1. When the PlantCARE database software is used for prediction and analysis, the length of the submitted sequence should not exceed 1500 bp, otherwise an error message will appear.
2. After using the bioinformatics database software to analyze the corresponding results, further design relevant experiments to verify the predicted results.

