GRKs (G Protein-coupled Receptor Kinases)
Related Symbol Search List
Immunology Background
About GRKs (G Protein-coupled Receptor Kinases)
G protein-coupled receptors (GPCRs) constitute the largest family of membrane receptors in human physiology, are widely distributed in multiple cell types, contain more than 1,000 different members, and mediate most of the responses to hormones, neurotransmitters, sensory stimuli, and a variety of autocrine and paracrine factors. GRKs, known as G protein-coupled receptor kinases, are an important family of tyrosine kinases. They are mainly involved in the regulation of G protein-coupled receptor (GPCR) signaling.
GRKs are found in metazoans and mammals, the seven GRKs can be divided into three subfamilies based on overall structural organization and homology: GRK1 (rhodopsin kinase) and GRK7; GRK2 (βARK1) and GRK3 (βARK2); and GRK4, GRK5, and GRK6. The central catalytic domain is flanked by the N-terminal region and the C-terminal region, which contains the regulator of the G protein signaling (RGS) structural domain and the C-terminal lipid-binding structural domain. GRKs are serine/threonine kinases with a tripartite modular structure. Interestingly, the X-ray crystal structure of GRK2 suggests that it can Interestingly, the X-ray crystal structure of GRK2 suggests that it can simultaneously bind to the receptor, Gαq (through the RGS domain), and Gβγ (through a C-terminal pleckstrin homology domain), thus providing an effective mechanism to terminate signaling. mechanism to terminate signaling.
A critical component in modulating GRK function involves regulating the activity and cellular localization of GRKs. Phosphorylation appears to play an important role in regulating GRK activity. GRK2 phosphorylation by ERK1/2 inhibits GRK2 activity while phosphorylation by PKA, Src, and PKC results in increased activity. In contrast, GRK5 activity is attenuated by PKC phosphorylation but stimulated by autophosphorylation. GRK function is also regulated by interaction with a large number of additional proteins including G protein subunits, the GRK-interacting protein GIT1, caveolin-1, phosphoinositide 3-kinase, cytoskeletal proteins such as tubulin and actin, and various calcium-binding proteins.
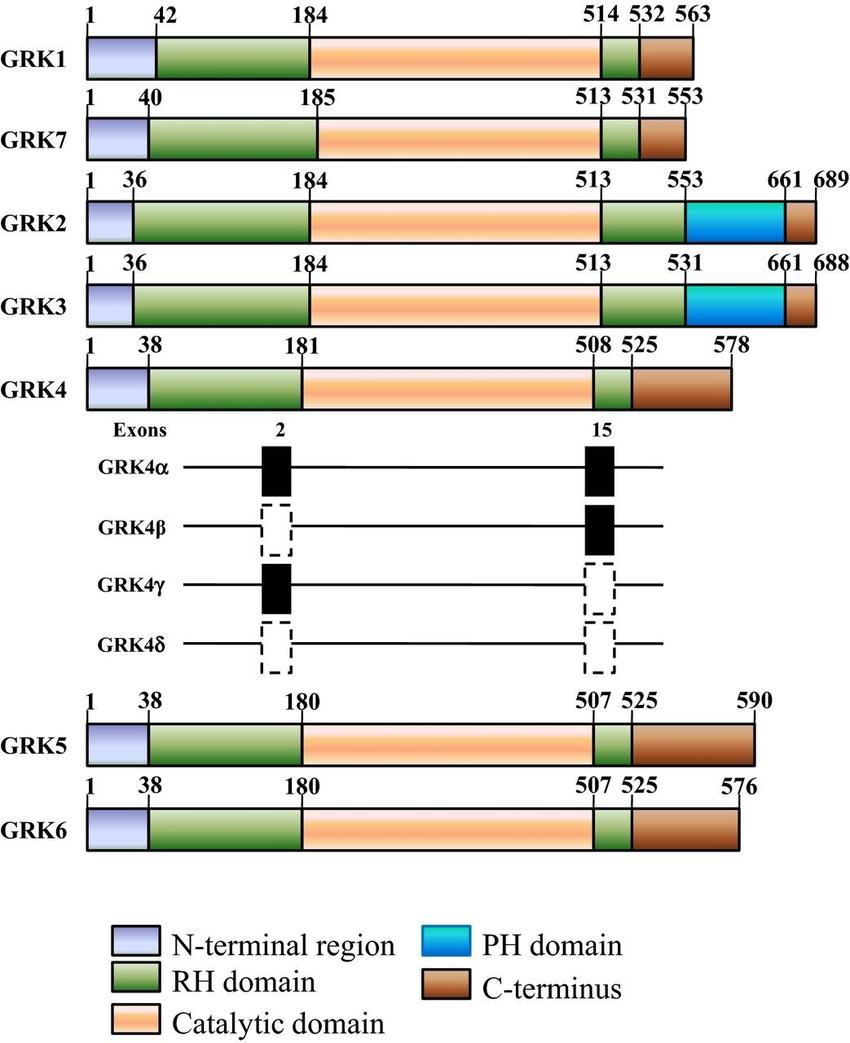 Fig.1 Structural domain distribution of GRKs. (Yang J, et al., 2016)
Fig.1 Structural domain distribution of GRKs. (Yang J, et al., 2016)
Mechanism of Action of GRKs
The mechanism of action of GRKs mainly involves the following aspects:
- GPCR Phosphorylation
GRKs stimulate receptor phosphorylation by reacting with activated GPCRs and transferring phosphate groups to specific tyrosine residues on the GPCR. This phosphorylation increases the affinity of the GPCR and further modulates its signaling and effects.
- β-Leucine Deactivation
Phosphorylated GPCRs can be bound by β-arrestins to separate them from G proteins, thus terminating the sudden appearance of GPCR signals. β-arrestins are also involved in the formation of internalized vesicles of GPCRs and are transported into the cell along with the internalized GPCRs.
- GPCR Internalization
GRKs-mediated phosphorylation prompts the formation of phosphorylated GPCRs in response to matching proteins, triggering the internalization of GPCRs. This internalization process can be achieved by intracellular transport mechanisms such as vesicles and fusion. GPCR internalization is essential for cellular response to stimuli and signal termination.
Through these mechanisms of action, GRK can precisely regulate the onset strength, duration, and spatial distribution of GPCR signaling to adapt to the needs of different cell types and environments. This is important for maintaining intracellular signaling homeostasis, regulating physiological functions, and participating in disease development.
Please note that the above is only a brief description of the mechanism of action of GRKs. the function and regulatory mechanism of GRKs may vary among different cell types and GPCR subtypes, so further studies are still necessary.
Functions of GRKs
- Cell Signaling
GRKs play important functions in cell signaling. GRKs regulate the signaling burst, internalization and de-activation, activity, and affinity of GPCRs, thereby affecting a variety of physiological processes including cell swelling, urinary, excretory, visual, neurotransmission, immune, and cardiovascular functions. Specific functions depend on GRK isoforms and specific cellular environments.
- Involvement in Disease Regulation
Abnormal activity of GRKs has been associated with the onset and progression of a variety of diseases. For example, GRKs play important roles in cardiovascular diseases, neurological disorders, kidney diseases, diabetes, and cancer. By modulating the negative regulation triggered by GPCR signaling, GRKs may be involved in regulating the aberrant activation or repair of disease-related signals, thereby affecting the development and progression of diseases. Therefore, GRKs have become an important target for the study and treatment of related diseases and can be used as a target to regulate the GPCR signaling pathway for the treatment of GPCR-related diseases.
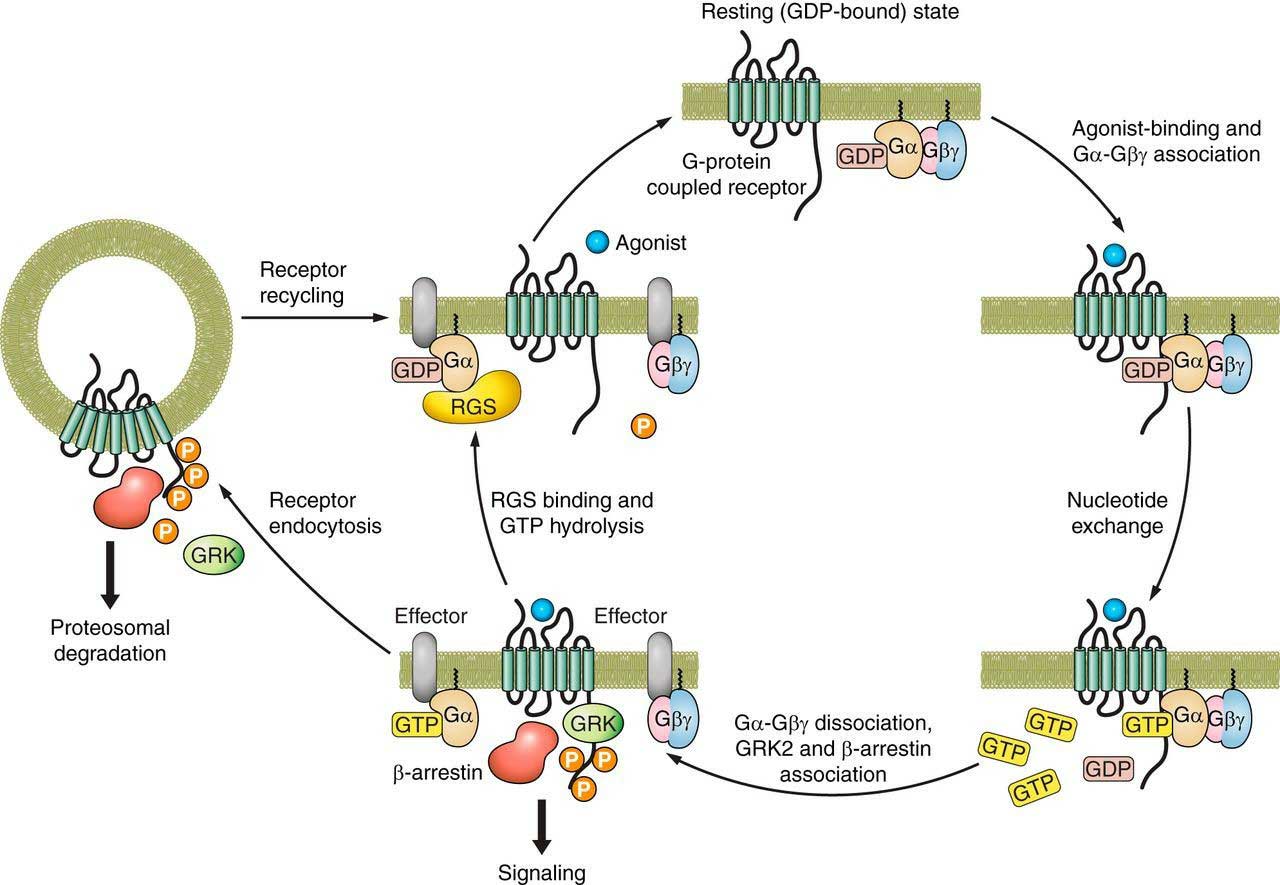 Fig.2 G protein-coupled receptor activation and reactivation. (Sato P Y, et al., 2015)
Fig.2 G protein-coupled receptor activation and reactivation. (Sato P Y, et al., 2015)
Available Resources for GRKs
Creative BioMart offers a variety of GRK-related research products, such as recombinant proteins, cell and tissue lysates, and protein pre-coupled magnetic beads, as well as customizable services and other resources to support your research in the field of GRKs. The following GRKs are displayed, click to view all related molecules/targets and research reagents. For further information or to purchase products, please contact us. We are committed to providing the highest quality resources and support for your research to help you succeed.
References:
- Yang J, Villar V A M, Armando I, et al. G protein-coupled receptor kinases: crucial regulators of blood pressure[J]. Journal of the American Heart Association, 2016, 5(7): e003519.
- Sato P Y, Chuprun J K, Schwartz M, et al. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease[J]. Physiological reviews, 2015, 95(2): 377-404.
- Laganà M, Schlecht-Louf G, Bachelerie F. The G Protein-Coupled Receptor Kinases (GRKs) in Chemokine Receptor-Mediated Immune Cell Migration: From Molecular Cues to Physiopathology. Cells. 2021;10(1):75.
- Ribas C, Penela P, Murga C, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 2007, 1768(4): 913-922.

