IVD of Betula verrucosa Allergy
🧪 BETVIII-2261B
Source: E.coli
Species: Betula Pendula
Tag: His&Myc&SUMO
Conjugation:
Protein Length: 1-205 aa
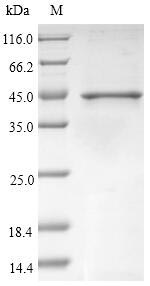
🧪 Bet v 1-09B
Source: Nicotiana Benthamiana
Species: Betula pendula
Tag: His
Conjugation:
Protein Length:
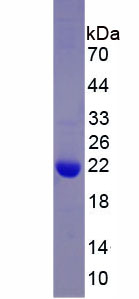
🧪 BETVIA-3891B
Source: E.coli
Species: Betula verrucosa
Tag: His&SUMO
Conjugation:
Protein Length: 2-160aa

🧪 BETVII-4001B
Source: E.coli
Species: Betula verrucosa
Tag: His&SUMO
Conjugation:
Protein Length: 2-133aa

🧪 BETVIA-2719S
Source: E.coli
Species: Spinach
Tag: His
Conjugation:
Protein Length: Gly2-Asn160
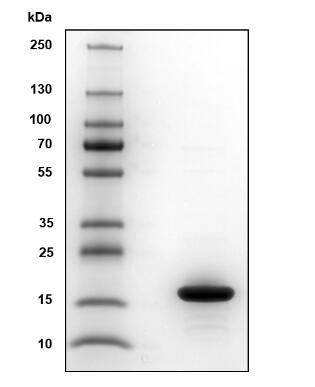
🧪 BETVII-01H
Source: E.coli
Species: Betula Pendula
Tag: His
Conjugation:
Protein Length: 141

🧪 BETVIA-1640B
Source: E.coli
Species: Betula pendula
Tag: His
Conjugation:
Protein Length: Met1-Asn160

🧪 BETVII-1641B
Source: E.coli
Species: Betula pendula
Tag: His
Conjugation:
Protein Length: Met1-Leu133

Betula verrucosa
Betula verrucosa is a prevalent allergen that can be inhaled, causing allergic reactions when the human body comes into contact with or ingests specific components of this substance. Current research shows that allergy is primarily related to pollen. The pollen of birch bark is highly allergenic due to its large inflorescences and easy dispersal of pollen. Allergy sufferers may experience allergic reactions such as asthma, allergic rhinitis, and conjunctivitis. Currently, in vitro diagnostics (IVD) for birch allergy usually require a series of allergy tests, including skin prick testing and serum IgE testing.

Main Steps of IVD for Betula verrucosa Allergy
IVD for Betula verrucosa allergy is usually achieved by detecting specific IgE antibodies against Betula verrucosa pollen in the patient's serum.
- Western Blotting
This diagnostic method detects IgE antibodies at the protein level. The Betula verrucosa pollen extract was subjected to SDS-PAGE electrophoresis, then transferred to a membrane, reacted with the patient's serum, and finally developed with enzyme-labeled secondary antibodies.
- Immunohistochemistry
This method involves fixing Betula verrucosa pollen extract on a glass slide, reacting it with the patient's serum, and then adding fluorescently labeled secondary antibodies for color development. This method can detect the presence of specific IgE antibodies against Betula verrucosa pollen in patient serum and allows for semi-quantitative analysis of allergens in pollen extracts.
Creative BioMart provides high-quality recombinant Betula verrucosa allergen proteins used for IVD, including ELISA, lateral flow assay, western blot, and other immunoassays.
Highlights of Our Products
- High purity. Produced in cell culture or microorganisms through genetic engineering, this purification process is relatively simple and scalable.
- Good stability. Compared with natural allergens, recombinant allergens are more resistant to heat, acid, and alkali, and are less susceptible to denaturation and inactivation.
- Good quality and low price. Large-scale production also reduces production costs.
- Higher safety and repeatability.
Our Outstanding Advantages
- We own a proficient technical team, top-of-the-line scientific research equipment, and cutting-edge technology to provide unparalleled services.
- Our diverse range of IVD products caters to the varied needs of customers, and we strive to offer comprehensive scientific research support.
- We place utmost importance on service quality, with a commitment to providing timely and precise IVD-related services, ensuring our customers receive an optimal scientific research experience.
Clinical Related Information
The major allergen of birch pollen is Bet v 1. Birches, especially Betula pendula and Betula verrucosa, release this protein, which can trigger allergic reactions in susceptible individuals.
Symptoms of Birch Pollen Allergy
People with a birch pollen allergy may experience a range of symptoms, including:
Sneezing; Runny or stuffy nose (allergic rhinitis); Itchy and watery eyes; Itchy mouth or throat; Coughing; Fatigue; Asthma-like symptoms in more severe cases.
How to Overcome Birch Pollen Allergy
To manage and mitigate symptoms of birch pollen allergy, the following strategies can be helpful:
Medications:
- Antihistamines: To reduce symptoms such as sneezing, runny nose, and itching.
- Nasal corticosteroids: For inflammation and congestion.
- Leukotriene inhibitors: To help relieve nasal symptoms.
- Decongestants: To reduce nasal congestion.
Allergy Immunotherapy:
- Subcutaneous Immunotherapy (SCIT): Allergy shots that help build tolerance to allergens.
- Sublingual Immunotherapy (SLIT): Allergy tablets or drops placed under the tongue.
Avoidance Measures:
- Stay indoors during peak pollen times (usually early morning and on windy days).
- Keep windows and doors closed during high pollen seasons.
- Use high-efficiency particulate air (HEPA) filters in your home.
- Wash hair and change clothes after being outdoors.
- Avoid outdoor activities when pollen counts are high.
Saline Nasal Irrigation: Using saline solutions to rinse off pollen from nasal passages can provide relief.
Foods to Avoid if Allergic to Birch Pollen
Some individuals with birch pollen allergy may experience oral allergy syndrome (OAS) or pollen-food syndrome, where certain fruits, vegetables, and nuts trigger allergic responses. Common foods to avoid include:
| Apples | Pears | Cherries | Plums |
| Peaches | Kiwi | Carrots | Celery |
| Potatoes | Hazelnuts | Almonds | Walnuts |
These reactions occur because the proteins in these foods are similar to the Bet v 1 protein found in birch pollen.
Case Study
Case 1: de Jong NW, Terlouw S, van Boven FE, van Maaren MS, Schreurs MWJ, van den Berg-Somhorst DBPM, Esser D, Bastiaan-Net S. Birch Pollen Related Pear Allergy: A Single-Blind Oral Challenge TRIAL with 2 Pear Cultivars. Nutrients. 2021 Apr 18;13(4):1355. doi: 10.3390/nu13041355. PMID: 33919631; PMCID: PMC8073155.
So far, in the literature, less attention has been given to Bet v 1 cross-reactive symptoms caused by pear (Pyrus communis). In the Netherlands, pears are widely consumed. The primary objective of this study was to measure the type and severity of allergic symptoms during pear challenges in birch pollen allergic patients, with a positive history of pear allergy, using two different pear varieties. The authors found a high likelihood of fewer and less severe symptoms during the 'Cepuna' challenges. Consequently selected pear sensitized patients can try to consume small doses of the 'Cepuna' pear outside the birch pollen season.
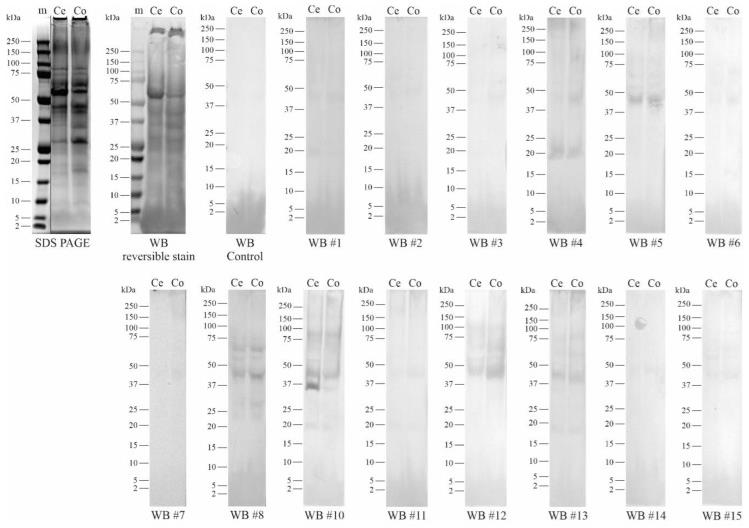 Fig2. Western blots (WB) of 'Cepuna' and 'Conference' pear total protein concentrate using the patient serum. The WB control blot was exposed to buffer instead of serum. Patient numbering is indicated by #.
Fig2. Western blots (WB) of 'Cepuna' and 'Conference' pear total protein concentrate using the patient serum. The WB control blot was exposed to buffer instead of serum. Patient numbering is indicated by #.Case 2: Raith M, Swoboda I. Birch pollen-The unpleasant herald of spring. Front Allergy. 2023 May 15;4:1181675. doi: 10.3389/falgy.2023.1181675. PMID: 37255542; PMCID: PMC10225653.
While the most important allergenic molecules of birch pollen have been identified and characterized, the contribution of other pollen components, such as lipids, non-allergenic immunomodulatory proteins, or the pollen microbiome, to the development of allergic reactions are sparsely known. Furthermore, what also needs to be considered is that pollen is exposed to external influences which can alter its allergenicity. The author focus on epithelial cells since these cells are the first line of defense in respiratory disease and are increasingly considered to be a regulatory tissue for the protection against the development of respiratory allergies.
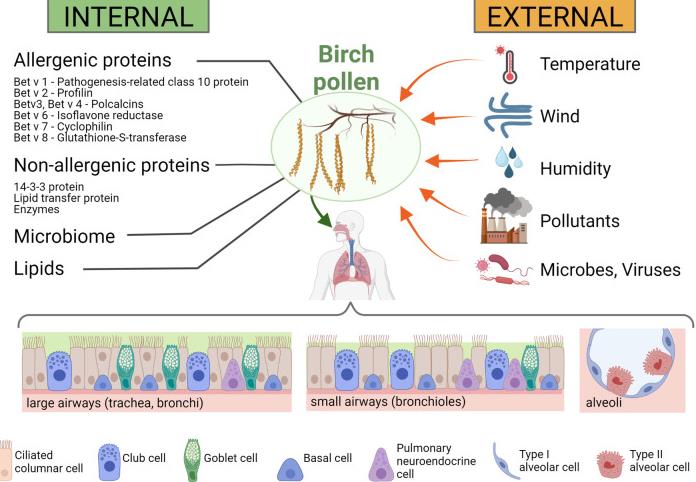 Fig3. Internal and external influences on birch pollen and the pollen's interaction with the human respiratory tract. Internal influences on the immunomodulatory effects of birch pollen (left panel): Birch pollen contains a number of allergenic proteins, such as Bet v 1 (a pathogenesis-related class 10 protein), Bet v 2 (a profilin), Bet v 3 and Bet v 4 (both polcalcins), Bet v 6 (an isoflavone reductase), Bet v 7 (a cyclophilin) and Bet v 8 (a glutathione-S-transferase), but also non-allergenic immunomodulatory proteins such as 14-3-3 protein, lipid transfer proteins and a number of enzymes. In addition, also lipids are present in high amounts in birch pollen, and microbes (such as proteobacteria or actinobacteria) and viruses (such as idaeovirus or cherry leaf roll virus) (85) are found in the pollen coat. External influences on birch pollen and their immunomodulatory effects (right panel): Meteorological influences such as temperature, wind and humidity can affect the immunomodulatory activity of the pollen and the pollen load, but other biotic (e.g., microbes and viruses) and abiotic (e.g., air pollutants) factors can also affect the allergenicity of the pollen. Lower panel: Respiratory cells that interact with the birch pollen. The cellular composition of the airway epithelium changes throughout the respiratory tract, with the large airways (trachea, bronchi) having higher number of goblet cells than the small airways (bronchioles) or the alveoli. Created with BioRender.com.
Fig3. Internal and external influences on birch pollen and the pollen's interaction with the human respiratory tract. Internal influences on the immunomodulatory effects of birch pollen (left panel): Birch pollen contains a number of allergenic proteins, such as Bet v 1 (a pathogenesis-related class 10 protein), Bet v 2 (a profilin), Bet v 3 and Bet v 4 (both polcalcins), Bet v 6 (an isoflavone reductase), Bet v 7 (a cyclophilin) and Bet v 8 (a glutathione-S-transferase), but also non-allergenic immunomodulatory proteins such as 14-3-3 protein, lipid transfer proteins and a number of enzymes. In addition, also lipids are present in high amounts in birch pollen, and microbes (such as proteobacteria or actinobacteria) and viruses (such as idaeovirus or cherry leaf roll virus) (85) are found in the pollen coat. External influences on birch pollen and their immunomodulatory effects (right panel): Meteorological influences such as temperature, wind and humidity can affect the immunomodulatory activity of the pollen and the pollen load, but other biotic (e.g., microbes and viruses) and abiotic (e.g., air pollutants) factors can also affect the allergenicity of the pollen. Lower panel: Respiratory cells that interact with the birch pollen. The cellular composition of the airway epithelium changes throughout the respiratory tract, with the large airways (trachea, bronchi) having higher number of goblet cells than the small airways (bronchioles) or the alveoli. Created with BioRender.com.Case 3: Wang X, Chen L, Ding J, Wang H, Wang X. Profiles of Birch Allergen Component Sensitization and Its Association with Pollen Food Allergy Syndrome in Northern China. J Asthma Allergy. 2023 Nov 14;16:1241-1250. doi: 10.2147/JAA.S427764. PMID: 38022747; PMCID: PMC10656847.
To investigate the major allergen components associated with birch pollen allergy in northern China and elucidate clinical relevance to pollen food allergy syndrome (PFAS). The predominant sensitization pattern is mono-sensitization to Bet v 1, but when considering immunotherapy, Bet v 2 should also be taken into account. Bet v 1 serves as a valuable biomarker for diagnosing PFAS and apple allergy.
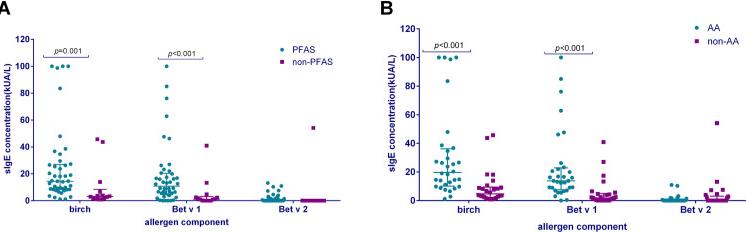 Fig4. Differences of sIgE levels of birch pollen and its allergen components in participants with food allergy and those not.
Fig4. Differences of sIgE levels of birch pollen and its allergen components in participants with food allergy and those not.





