The Wingless-INT (WNT) Signaling Pathway
- Related links
- Recombinant Proteins
- Native Proteins
- GMP Proteins
- Fluorescent Dyes
- The Wingless-INT (WNT) Signaling Pathway Information
- The Wingless-INT (WNT) Signaling Pathway
- Wnt Related Diseases
WNT Related Product
The Wingless-INT (WNT) signaling pathway is a similarly ancient developmental mechanism. All multicellular eukaryotic lineages (metazoans) express WNT proteins, including sponges. This predates even FGF’s ancient arrival, arising at or prior to the development of true multi-cellularity, far before the emergence of bilateral symmetry, three embryonic germ layers, and the divergence of protostomes and deuterostomes.
There are many WNT ligands encoded in the mouse and human genome, and multiple receptors. All WNT ligands are defined by their sequence similarity, specifically by the presence of 22 conserved cysteine residues, which are important for the formation of the protein’s secondary structure. The recent elucidation of the structure of a WNT ligand bound to a Frizzled (FZD) receptor has shed light on the many mysteries of WNT structure including that these cysteine residues, once hypothesized to be involved in intermolecular binding - perhaps by forming WNT dimers - are mostly involved in internal disulfide bonds.
WNT proteins undergo multiple post-translational modifications prior to their secretion, mostly glycosylation and acylation. Glycosylation modifications vary between different WNT ligands with variable effects on their activity and ability to be secreted. Acylation, on the other hand, is essential for the secretion of all WNT ligands. The acylation of WNT proteins is accomplished by the membrane bound O-acyl transferase (MBOAT) protein Porcupine (PORCN), originally identified as a segment polarity gene during Drosophila development. These fatty acid modifications contribute to the relative hydrophobicity of native WNT proteins. In fact, based solely on their amino acid sequence, WNT proteins should be very hydrophilic, as they have a number of charged residues and a predicted isoelectric point of 9. As a result, there are many theories about how WNT ligands travel through the extracellular space. In Drosophila, the Wnt homologue Wingless (Wg) has been shown to associate with lipid rafts, much like the apoB proteins in mammals. There are many other extracellular proteins that could also serve to transport WNT over long distances, including HSPGs and even potentially the WNT inhibitory protein sFRP (secreted frizzled receptor protein).
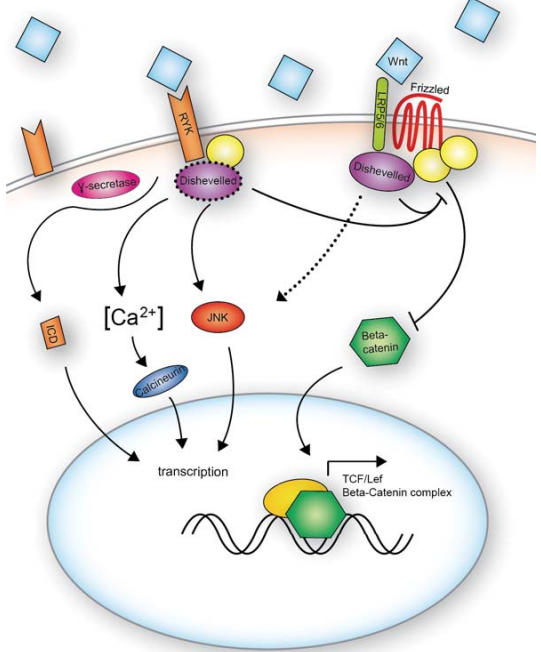
The usual receptor for the WNT ligands is one of the FZD family of receptors, of which there are 10 in humans and mice. FZD receptors are G-coupled receptors with a conserved extracellular cysteine rich domain (CRD), that binds to WNT ligands. The recently solved structure of a WNT ligand bound to a FZD receptor confirmed that there are two domains on the WNT protein that interact with the frizzled CRD. One of these WNT domains is thought to be palmitoylated in many different WNTs. Based on the structure of the FZD bound WNT, there is reason to think there are little to no differential binding properties between various WNT ligands and receptors, though not much is known on this topic. As a result, although WNT signaling downstream can take canonical or non-canonical forms, this is likely a function of the cell that receives the signal, rather than the signal itself. One contributing factor may be the cellular complement of FZD co-receptors; including LRP proteins (5 and 6 in vertebrates), ROR1/2, or RYK. LRP proteins function in a multimeric structure with various FZD receptors, and are phosphorylated in response to WNT binding. ROR1/2 and RYK, in contrast, while they also have confirmed or hypothesized co-receptor activity, also each contain an extracellular domain that binds directly to WNT and can act as receptors for WNT in the absence of FZD. The ROR1/2 WNT binding domain is similar to the CRD of the FZD receptors; RYK, however, contains a domain similar to that of the WNT antagonist WNT inhibitor factor (WIF). Despite the differences between these receptors, interestingly, they are thought to interact with similar portions of the WNT ligand.
Canonical WNT signaling downstream of FZD receptors regulates the level of the intracellular signaling molecule β-catenin. Under basal conditions, β-catenin is targeted for degradation by GSK3β. When WNT is bound to the FZD-LRP multimeric complex, a conformational change in the FZD intracellular domain results in the subsequent phosphorylation of LRP. As a result, adaptor proteins such as axin are recruited to the complex. These changes lead to a decrease in the activity of GSK3β and the degradation of β-catenin. Ultimately, the accumulation of β-catenin in the cell leads to the activation of TCF/LEF transcription factors and the transcription of their target, WNT-responsive, genes. As with most signaling cascades, many of these target genes are part of the signaling machinery itself, so the activation of TCF/LEF transcription factors further leads to the transcription and production of more TCF/LEF transcription factors.
As a β-catenin independent (thus non-canonical) WNT receptor, RYK is an interesting case. While similar to receptor tyrosine kinases, RYK’s intracellular tyrosine kinase domain is functionally dead. In the absence of a functional kinase domain, it was hypothesized that RYK would function primarily as a co-receptor. Recent studies have shown, however, that RYK’s intracellular domain can be cleaved and participate in transcriptional regulation following its translocation to the nucleus. While the cleavage event, mediated through γ-secretase activity, seems not to be dependent on WNT signaling, the nuclear translocation of the cleaved intracellular domain appears to require a WNT signal.
Wnt Signaling Within the Cytoplasm
Wnt pathway activation results in the elevation of cytoplasmic β-catenin protein levels in the cytoplasm. In the absence of the activation, β-catenin is phosphorylated by two kinases, casein kinase I and GSK-3. This interaction is facilitated by the scaffolding proteins, axin and adenomatous polyposis coli (APC). Together, these proteins form a degradation complex and targeted for ubiquitination and degradation by the proteasome.
In case of the Wnt pathway activation, elevation of β-catenin levels leads to β-catenin’s nuclear accumulation. Dsh can interact with the destruction complex and prevent the phosphorylation of β-catenin. Dsh, APC, GSK-3 and β-catenin participate in the signaling events in which axin is a limiting component of the Wnt signaling cascade. Axin acts as a key scaffolding molecule and participates in the rapid assembly and disassembly of Wnt pathway components to regulate β-catenin stability in the cell.
Wnt Signaling Within the Nucleus
The elevated levels of β-catenin following Wnt signaling leads to the transcriptional activation of target genes which interact with Tcf/Lef DNA-binding proteins. In the absence of the Wnt signal, TCF acts as a repressor of Wnt target genes by forming a complex with groucho, a family of transcriptional corepressor proteins in vertebrates and invertebrates. Once inside the nucleus, β-catenin converts the TCF repressor complex into a transcriptional activator complex. The activator complex requires a recruitment of the histone acetylase CBP/p300 (cyclic AMP response element-binding protein). CBP binds to the β-catenin/TCF complex as a coactivator. Another activator Brg-1, along with CBP, induces chromatin remodeling that facilitates target gene transcription.
The events occurring during Wnt signaling in the nucleus are controlled by number of protein molecules. The protein chibby binds to the C-terminus of β-catenin and acts a nuclear antagonist. Another protein ICAT carries out the dissociation of complexes between β-catenin, LEF and CBP/p300 by blocking the binding of β-catenin to TCF. The presence of these additional β-catenin-binding molecules raises the layer of complexity to the regulation of gene expression by nuclear β-catenin.

