Co-stimulatory ILT/CD85 Family Proteins
Related Symbol Search List
Immunology Background
Background
About Co-stimulatory ILT/CD85 Family Proteins
Ig-like transcripts (leukocyte Ig-like receptor/monocyte/macrophage Ig-like receptor) are encoded on human chromosome 19q13.4, designated the human leukocyte receptor complex, and are predominantly expressed on myeloid lineage cells. The ILT/CD85 family is involved in immune regulation and belongs to the immunoglobulin-like transcript (ILT) or leukocyte immunoglobulin-like receptor (LILR) family. These receptors are also known as CD85 proteins due to their classification as clusters of differentiation (CD) antigens.
The ILT/CD85 family members are characterized by their structural features, including extracellular immunoglobulin-like domains and cytoplasmic signaling motifs. They are predominantly expressed on immune cells, such as monocytes, macrophages, dendritic cells, and subsets of T and B cells.
While some ILT/CD85 receptors deliver inhibitory signals to regulate immune responses, there are certain family members that possess co-stimulatory properties, enhancing immune activation. This co-stimulatory function distinguishes them from other inhibitory receptors in the ILT/CD85 family.
These co-stimulatory ILT/CD85 family members contribute to the fine-tuning of immune responses and the maintenance of immune homeostasis. By providing positive signals, they enhance immune activation and modulate the balance between immune activation and regulation.
The specific ligands and signaling pathways associated with co-stimulatory ILT/CD85 receptors are still under investigation. Further research is needed to fully understand their functional implications and potential therapeutic applications in immune-related diseases and disorders.
It's important to note that while some ILT/CD85 family members have co-stimulatory properties, the majority of ILT/CD85 receptors function as inhibitory receptors, dampening immune responses to prevent excessive inflammation and maintain immune tolerance.
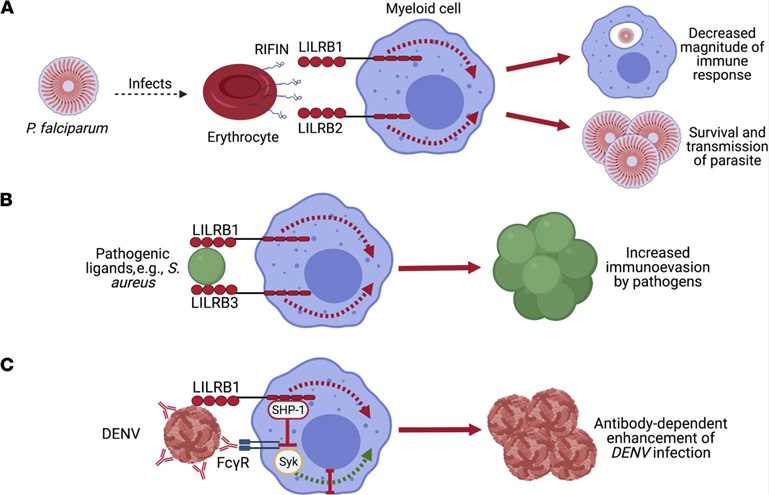 Fig.1 The interaction of pathogenic ligands with LILRBs promotes immunoevasion. (De Louche CD, et al., 2022)
Fig.1 The interaction of pathogenic ligands with LILRBs promotes immunoevasion. (De Louche CD, et al., 2022)Structural Domains of ILT/CD85 Family Proteins
The ILT/CD85 family proteins share a common structural organization, which includes extracellular immunoglobulin-like domains and cytoplasmic signaling motifs. Here is a breakdown of their structural features:
| Structural domains | Functions | |
|---|---|---|
| Extracellular Immunoglobulin-like Domains |
|
|
| Cytoplasmic Signaling Motifs |
|
|
| Ligands and Receptor-Ligand Interactions |
|
|
Understanding the structural characteristics of ILT/CD85 family proteins is crucial for deciphering their roles in immune regulation, immune cell activation, and potential therapeutic applications in immune-related diseases.
Co-stimulatory and Co-inhibitory Pathways of the ILT/CD85 Family Proteins
The ILT/CD85 family proteins encompass both co-stimulatory and co-inhibitory receptors that can modulate immune responses through specific signaling pathways. Here's an overview of the co-stimulatory and co-inhibitory pathways associated with ILT/CD85 family proteins:
| Pathways | Proteins and functions | ||
|---|---|---|---|
| Co-stimulatory Pathways | LILRB4 (ILT3) Co-stimulatory Pathway |
|
|
| LILRA4 (ILT7) Co-stimulatory Pathway |
|
||
| Co-inhibitory Pathways | LILRB1 (ILT2) Co-inhibitory Pathway |
|
|
| ILT4 (LILRB2) Co-inhibitory Pathway |
|
||
These co-stimulatory and co-inhibitory pathways of ILT/CD85 family proteins play crucial roles in regulating immune responses, maintaining immune homeostasis, and influencing the outcome of various diseases, including cancer, autoimmune disorders, and transplantation. The balance between co-stimulatory and co-inhibitory signals mediated by ILT/CD85 family members is essential for proper immune function.
ILT/CD85 Family Proteins
ILT/CD85 family members include LILRA3, LILRB1, ILT4, LILRB3, LILRB4, LILRA4, and others. Some of these are co-stimulatory receptors and some are inhibitory receptors. The following is an overview of these proteins:
| Key molecules | Functions |
|---|---|
| LILRA3 |
|
| LILRB1 |
|
| ILT4 |
|
| LILRB3 |
|
| LILRB4 |
|
| LILRA4 |
|
These ILT/CD85 family proteins play diverse roles in immune regulation, immune cell activation, and immune tolerance. The balance between co-stimulatory and inhibitory receptors within the ILT/CD85 family is crucial for maintaining proper immune responses and immune homeostasis.
Role of ILT/CD85 Family Proteins in Different Diseases
The ILT/CD85 family proteins encompass both co-stimulatory and co-inhibitory receptors, and their roles in different diseases can be diverse. Here's an overview of the roles of co-stimulatory and co-inhibitory ILT/CD85 family proteins in various diseases:
Cancer
- Co-stimulatory ILT/CD85 family proteins, such as LILRB4 (ILT3), can enhance anti-tumor immune responses by promoting T cell activation and proliferation.
- Conversely, co-inhibitory ILT/CD85 family proteins, including LILRB1 (ILT2) and ILT4 (LILRB2), can dampen immune responses and contribute to immune evasion by tumors.
- The balance between co-stimulatory and co-inhibitory ILT/CD85 family members influences the immune response to cancer and can be targeted for immunotherapeutic interventions.
Autoimmune Disorders
- Dysregulation of ILT/CD85 family proteins can contribute to the pathogenesis of autoimmune disorders.
- Co-inhibitory ILT/CD85 family members like LILRB1 and ILT4 can impair immune tolerance and promote autoimmunity by suppressing immune cell activation and inhibiting the clearance of self-reactive cells.
- On the other hand, co-stimulatory ILT/CD85 family proteins, such as LILRB4, can enhance immune responses and potentially exacerbate autoimmune conditions.
Infectious Diseases
- ILT/CD85 family proteins, both co-stimulatory and co-inhibitory, play roles in modulating immune responses during infectious diseases.
- Co-stimulatory ILT/CD85 family proteins, like LILRB4 and LILRA4 (ILT7), can enhance immune cell activation and contribute to the control of viral infections.
- Co-inhibitory ILT/CD85 family proteins, such as LILRB1 and ILT4, can modulate immune responses to prevent excessive inflammation but may also impair immune clearance of pathogens.
Transplantation
- The ILT/CD85 family proteins have been implicated in regulating immune responses in transplantation settings.
- Co-inhibitory ILT/CD85 family members like LILRB1 and ILT4 are involved in immune tolerance induction and can suppress immune cell activation to prevent graft rejection.
- Co-stimulatory ILT/CD85 family proteins, such as LILRB4, may impact graft acceptance or rejection by influencing immune cell activation and the balance between tolerance and rejection.
It's important to note that the roles of ILT/CD85 family proteins in diseases can vary depending on the specific family members, their ligands, the cell types involved, and the disease context. Further research is needed to fully elucidate their contributions and explore their potential as therapeutic targets in different diseases.
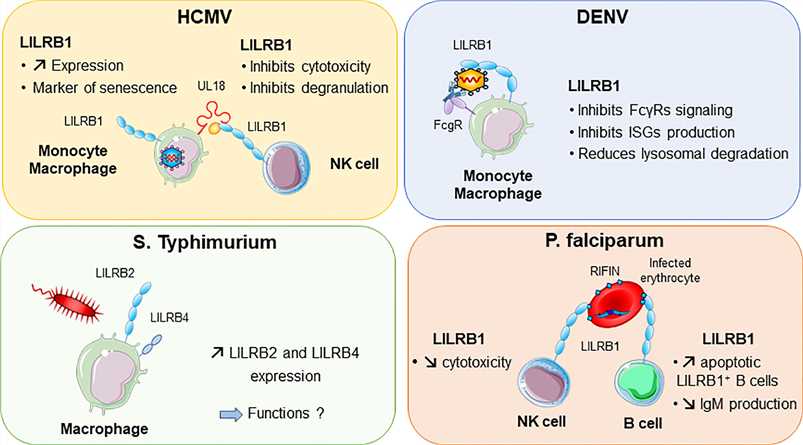 Fig.2 LILRB1 and LILRB2 implication in various infectious diseases. (Abdallah F, et al., 2021)
Fig.2 LILRB1 and LILRB2 implication in various infectious diseases. (Abdallah F, et al., 2021)Case Study
Case 1: Liu X, Yu X, Xie J, et al. ANGPTL2/LILRB2 signaling promotes the propagation of lung cancer cells. Oncotarget. 2015;6(25):21004-21015.
To examine the expression of LILRB2 in human lung cancer cell lines, the authors first assessed the expression of LILRB2 by flow cytometry in several NSCLC cell lines, including H1299, A549, H460 and H292G cells. H1299 and A549 cells highly expressed LILRB2 compared to other cancer cell lines and normal lung epithelial BEAS-2B cells evaluated (A, B) The authors confirmed that H1299, A549, and H292G cells had higher levels of LILRB2 protein expression by protein blotting (C).
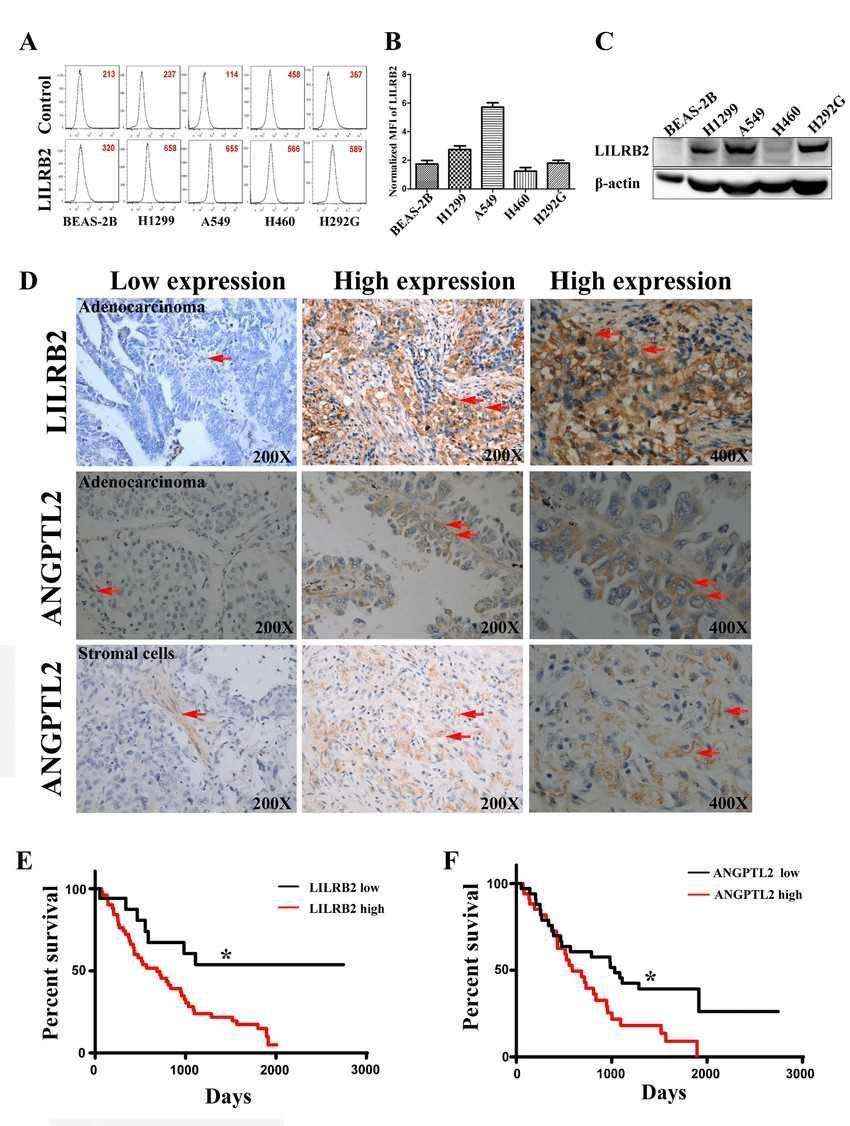 Fig.1 LILRB2 is highly expressed on human cancer cell lines and in primary tumor samples.
Fig.1 LILRB2 is highly expressed on human cancer cell lines and in primary tumor samples.Case 2: Deng M, Gui X, Kim J, et al. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature. 2018;562(7728):605-609.
The authors sought to determine the LILRB4 downstream signaling required for T cell suppression and leukemic infiltration. The phosphatases SHP-1, SHP-2, and SHIP can be recruited to the intracellular domain of LILRB 2. lilrb4 -KO AML cells phosphorylated SHP-2 at a lower level than wild-type cells but not SHP-1 or SHIP (a). deletion of SHP-2 (but not SHP-1 or SHIP) rescued T cell suppression in THP -1 cells from T cell suppression (b) and reduced short-term (20 h) and long-term (21 days) infiltration of THP-1 cells (c-d). Our results suggest that SHP-2 is a mediator of LILRB4 signaling.
Ingenuity Pathway analysis showed that the activities of the key transcription factors NFkB1 and RELA in the NF-κB pathway (positively regulated by SHP-2) were most significantly inhibited by the absence of lilrb4 (e). In lilrb4 -KO AML cells, phosphorylation of IKKα/β and levels of nuclear NF-κB were consistently reduced (f-g). Inhibition of NF-κB signaling restores T cell suppression and reduces AML cell infiltration in a LILRB4-dependent manner (h-i).
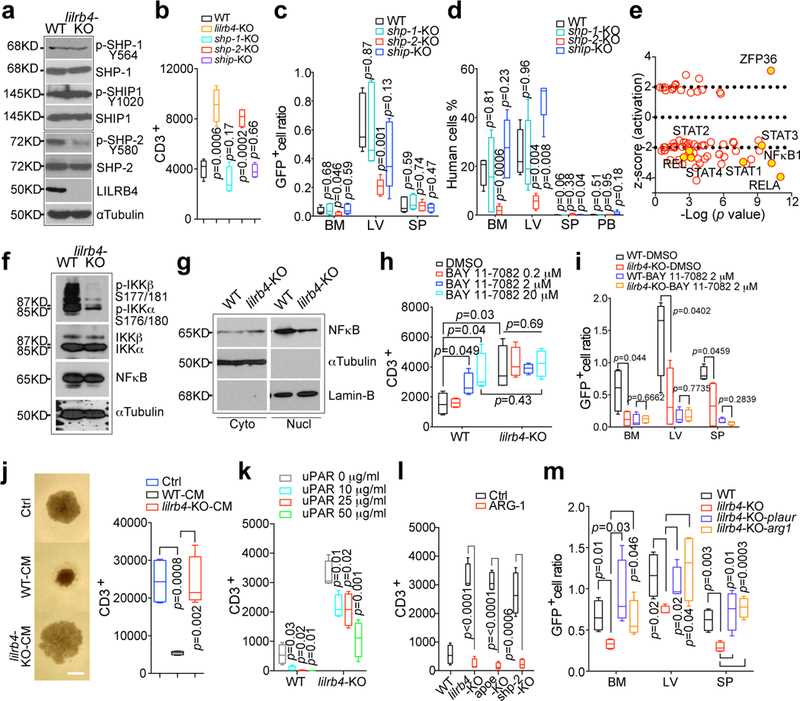 Fig.2 LILRB4-mediated intracellular signaling controls AML cell migration and T cell suppression.
Fig.2 LILRB4-mediated intracellular signaling controls AML cell migration and T cell suppression.References
- De Louche CD, Roghanian A. Human inhibitory leukocyte Ig-like receptors: from immunotolerance to immunotherapy. JCI Insight. 2022;7(2):e151553.
- Abdallah F, Coindre S, Gardet M, et al. Leukocyte immunoglobulin-like receptors in regulating the immune response in infectious diseases: A window of opportunity to pathogen persistence and a sound target in therapeutics. Front Immunol. 2021;12:717998.
- Chen X, Yuan M, Zhong T, et al. LILRB2 inhibition enhances radiation sensitivity in non-small cell lung cancer by attenuating radiation-induced senescence. Cancer Lett. 2024;593:216930.

