SLAM Family
Related Symbol Search List
Immunology Background
Background
About SLAM Family
The SLAM (Signaling Lymphocyte Activation Molecule) family is a group of cell surface receptors involved in immune cell activation and regulation. SLAM family receptors are primarily expressed on immune cells, including T cells, B cells, natural killer (NK) cells, and dendritic cells. They play important roles in immune cell signaling, immune cell interactions, and modulating immune responses. The SLAM family is named after the prototypical member, CD150 or SLAMF1.
The SLAM family includes several members, such as SLAMF1 (CD150), SLAMF2 (CD48), SLAMF3 (CD229), SLAMF4 (CD244), SLAMF5 (CD84), SLAMF6 (CD352), SLAMF7 (CD319), and SLAMF8 (CD353), and others. Each member of the SLAM family has distinct expression patterns and functions, but they share structural similarities. IgV (V)- and IgC2 (C2)-like domains are found in the extracellular areas. Via the IgV-like domain, the receptors attach to self-ligands with great affinity. The cytoplasmic tail of every family member, except for SLAMF2, SLAMF8, and SLAMF9, has an immunoreceptor tyrosine-based switch motif (ITSM). SLAMF4 has the highest number of ITSM motifs. SLAMF3 has four Ig-like domains in its extracellular domain.
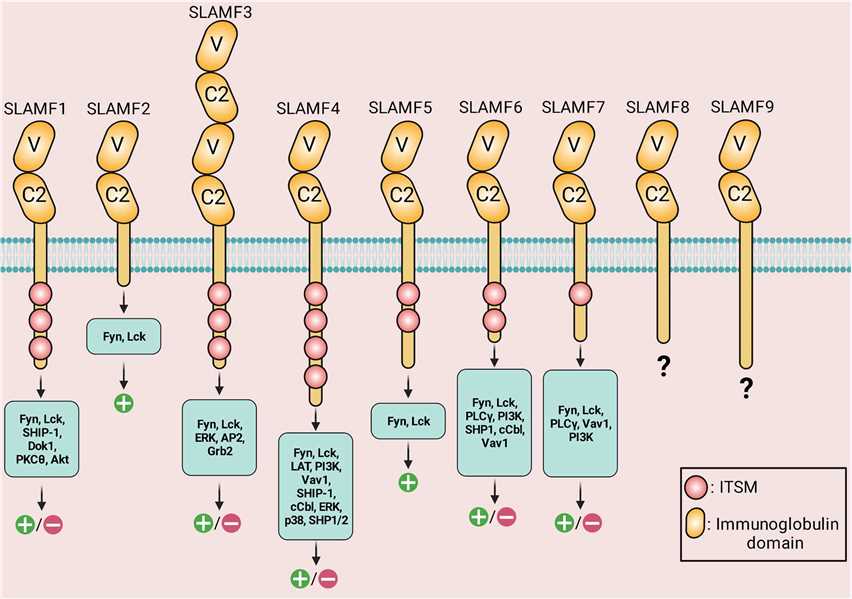 Fig.1 Structure, downstream signaling molecules, and the known signaling outcomes of SLAM-family receptors. Positive symbols and negative symbols indicate activatory outcome and inhibitory outcome, respectively. (Farhangnia P, et al., 2023)
Fig.1 Structure, downstream signaling molecules, and the known signaling outcomes of SLAM-family receptors. Positive symbols and negative symbols indicate activatory outcome and inhibitory outcome, respectively. (Farhangnia P, et al., 2023)The Key Roles of the SLAM Family
The SLAM family plays crucial roles in immune cell activation, signaling, and cell-cell interactions. Here are the key roles of the SLAM family in these processes:
| The key roles | Details | |
|---|---|---|
| Immune Cell Activation |
|
|
| Signaling Pathways |
|
|
| Immune Cell Interactions |
|
|
| Co-stimulation and Co-inhibition |
|
|
| Immune Cell Differentiation and Function |
|
|
The SLAM family's involvement in immune cell activation, signaling, and cell-cell interactions has implications for various aspects of immune responses, including adaptive immunity, innate immunity, and immune cell-mediated cytotoxicity. Dysregulation or mutations in SLAM family members have been associated with immune disorders, autoimmune diseases, and cancer. Understanding the functions and signaling mechanisms of the SLAM family receptors provides insights into immune cell biology and may lead to the development of targeted therapies for immune-related diseases.
The SLAM Family Members
The SLAM family consists of several members, each with distinct functions and expression patterns. Here are some of the key members of the SLAM family:
| Key molecules | Functions and expression patterns |
|---|---|
| SLAMF1 (CD150) |
|
| SLAMF2 (CD48) |
|
| SLAMF3 (CD229) |
|
| SLAMF4 (CD244) |
|
| SLAMF5 (CD84) |
|
| SLAMF6 (CD352) |
|
| SLAMF7 (CD319) |
|
These are just a few examples of the SLAM family members. Other members, such as SLAMF8 (CD353), SLAMF6 (Ly108), and SLAMF9, have also been identified, each with unique roles and functions in immune cell biology. The diverse functions and interactions of SLAM family members contribute to the regulation and coordination of immune responses.
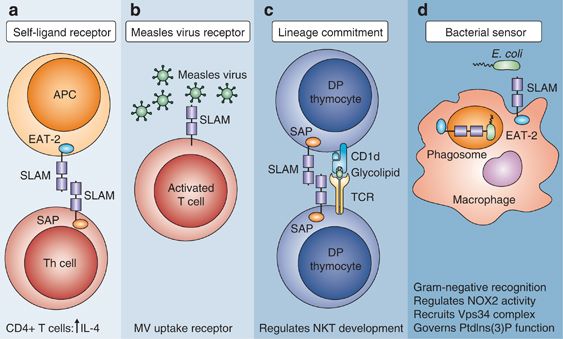 Fig.2 SLAM (CD150)-mediated functions in adaptive and innate immunity. (Sintes J, et al., 2011)
Fig.2 SLAM (CD150)-mediated functions in adaptive and innate immunity. (Sintes J, et al., 2011)The Potential Clinical Applications of the SLAM Family
The SLAM family and its members have shown potential clinical applications in immunity-related diseases and tumor immunotherapy. Here are some areas where the SLAM family is being explored:
Immunodeficiency Disorders
- Mutations in SLAMF1 (CD150) have been associated with X-linked lymphoproliferative syndrome (XLP), a rare immunodeficiency disorder.
- Understanding the role of SLAMF1 and other SLAM family members in immune cell activation and regulation can aid in the development of targeted therapies for immunodeficiency disorders.
Autoimmune Diseases
- SLAM family receptors, such as SLAMF5 (CD84) and SLAMF7 (CD319), have been implicated in autoimmune diseases like systemic lupus erythematosus (SLE).
- Modulating the activity of SLAM family receptors or their ligands may offer therapeutic strategies for autoimmune conditions.
Cancer Immunotherapy
- SLAM family receptors are being explored as targets for cancer immunotherapy due to their expression patterns on immune cells and their involvement in immune cell activation and cytotoxicity.
- SLAMF7 (CD319) is a therapeutic target in multiple myeloma, and monoclonal antibodies targeting SLAMF7, such as elotuzumab, have been developed for the treatment of this blood cancer.
- Manipulating SLAM family receptors and their signaling pathways may enhance immune cell-mediated anti-tumor responses.
Combination Therapies
- The SLAM family receptors and their ligands can be targeted in combination with other immunotherapeutic approaches to enhance treatment efficacy.
- For example, combining SLAM family-targeting antibodies with immune checkpoint inhibitors or chimeric antigen receptor (CAR) T cell therapies may provide synergistic effects in cancer treatment.
Vaccine Development
- SLAM family receptors, particularly SLAMF1 (CD150), play roles in immune cell activation and antigen presentation.
- Understanding the interactions between SLAM family receptors and pathogens can aid in the development of vaccines and adjuvants that enhance immune responses.
Exploring the roles and therapeutic potential of the SLAM family in immunity-related diseases and tumor immunotherapy is an active area of research. Further studies and clinical trials are needed to evaluate the efficacy and safety of targeting SLAM family receptors and their ligands in specific disease contexts.
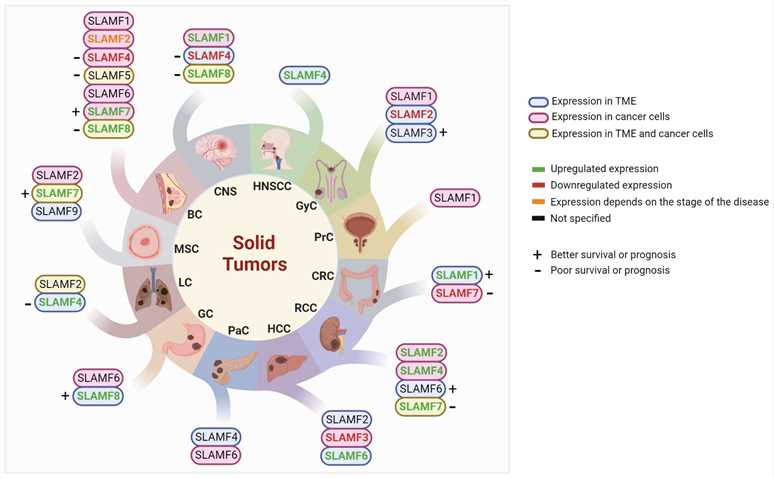 Fig.3 Studied SLAMF receptors in solid tumors. (Gunes M, et al., 2024)
Fig.3 Studied SLAMF receptors in solid tumors. (Gunes M, et al., 2024)Case Study
Case 1:Hajaj E, Eisenberg G, Klein S, et al. SLAMF6 deficiency augments tumor killing and skews toward an effector phenotype revealing it as a novel T cell checkpoint. Elife. 2020;9:e52539.
The goal of the experiments was to identify mechanisms underlying the improved effector function of Pmel-1 x SLAMF6 -/- lymphocytes.
- The authors initially evaluated the level of SAP, the primary adaptor required for SLAMF6 signaling, encoded by the Sh2d1a gene, which was intact in the SLAMF6 -/- mice (A). SAP is a critical adaptor that recruits Fyn kinase to SLAMF6. However, while SAP transcript was found at similar levels in WT and SLAMF6 -/- lymphocytes, SAP protein was not detectable in SLAMF6 -/- cells (B).
- Next, the authors measured by flow cytometry the level of phosphorylated ribosomal protein S6 (rpS6), an integrator of important signaling pathways, including PI3K/AKT/mTOR and RAS-ERK (C). No difference in phosphorylated rpS6 was found in the Pmel-1 x SLAMF6 -/- T cells.
- To identify transcription regulators whose activity differed between the Pmel-1 cells and the Pmel-1 x SLAMF6 -/- cells. The most prominent regulator found was T-bet, which increased more than twofold in activated Pmel-1 x SLAMF6 -/- splenocytes, followed by Eomes (D).
- Lastly, the level of immune receptors that mediate exhaustion was recorded during prolonged activation. Knocking out SLAMF6 together with LAG-3 blockade resulted in a three-fold increase in IFN-γ production (F). To evaluate the combination of SLAMF6 -/- cells and LAG-3 blocking antibody, the authors conducted an ACT experiment in melanoma-bearing mice. The control arm consisted of Pmel-1 x SLAMF6 -/- CD8+ T cells stimulated and sustained with an isotype antibody (G–I).
These results demonstrate that blocking the compensatory rise of LAG-3 on SLAMF6-/- T cells improved their anti-tumor effect even further.
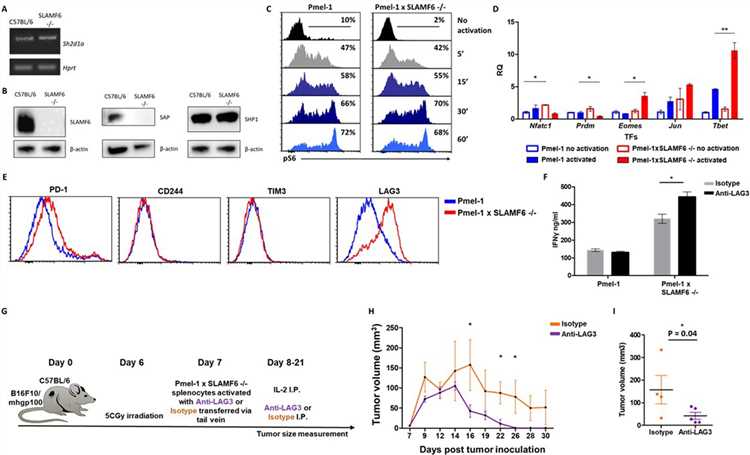 Fig.1 Mechanism associated with the inhibitory function of SLAMF6.
Fig.1 Mechanism associated with the inhibitory function of SLAMF6.Case 2: Sugimoto A, Kataoka TR, Ito H, et al. SLAM family member 8 is expressed in and enhances the growth of anaplastic large cell lymphoma. Sci Rep. 2020;10(1):2505.
The authors examined the expression of SLAMF8 mRNA and protein in the human ALCL cell lines SU-DHL-1 and Karpas299 using RT-PCR and immunoblotting, respectively. Both cell lines expressed SLAMF8 mRNA and protein (A, B). Both cell lines showed ALK translocation9,10 and the authors treated these with an ALK inhibitor, crizotinib11. The administration of crizotinib did not significantly affect the expression of SLAMF8 mRNA in either cell line (C) but significantly decreased the expression of SLAMF8 protein in both cell lines (D), after the authors confirmed the inhibitory effect of crizotinib on the phosphorylations of ALK in both cell lines (D).
Next, the authors examined the expression of SLAMF8 protein in human pathological specimens of non-neoplastic lymph nodes, non-neoplastic tonsils, and ALCL by immunohistochemistry. Lymphocytes, including non-neoplastic lymph node and non-neoplastic tonsil specimens, were negative for SLAMF8 protein and mRNA (E, F). Overall, 12 of 23 ALCL cases (52.2%) were positive for SLAMF8 protein and mRNA (E, F). SLAMF8 expression was not associated with age, gender, prognoses, ALK status, or lesion site (G).
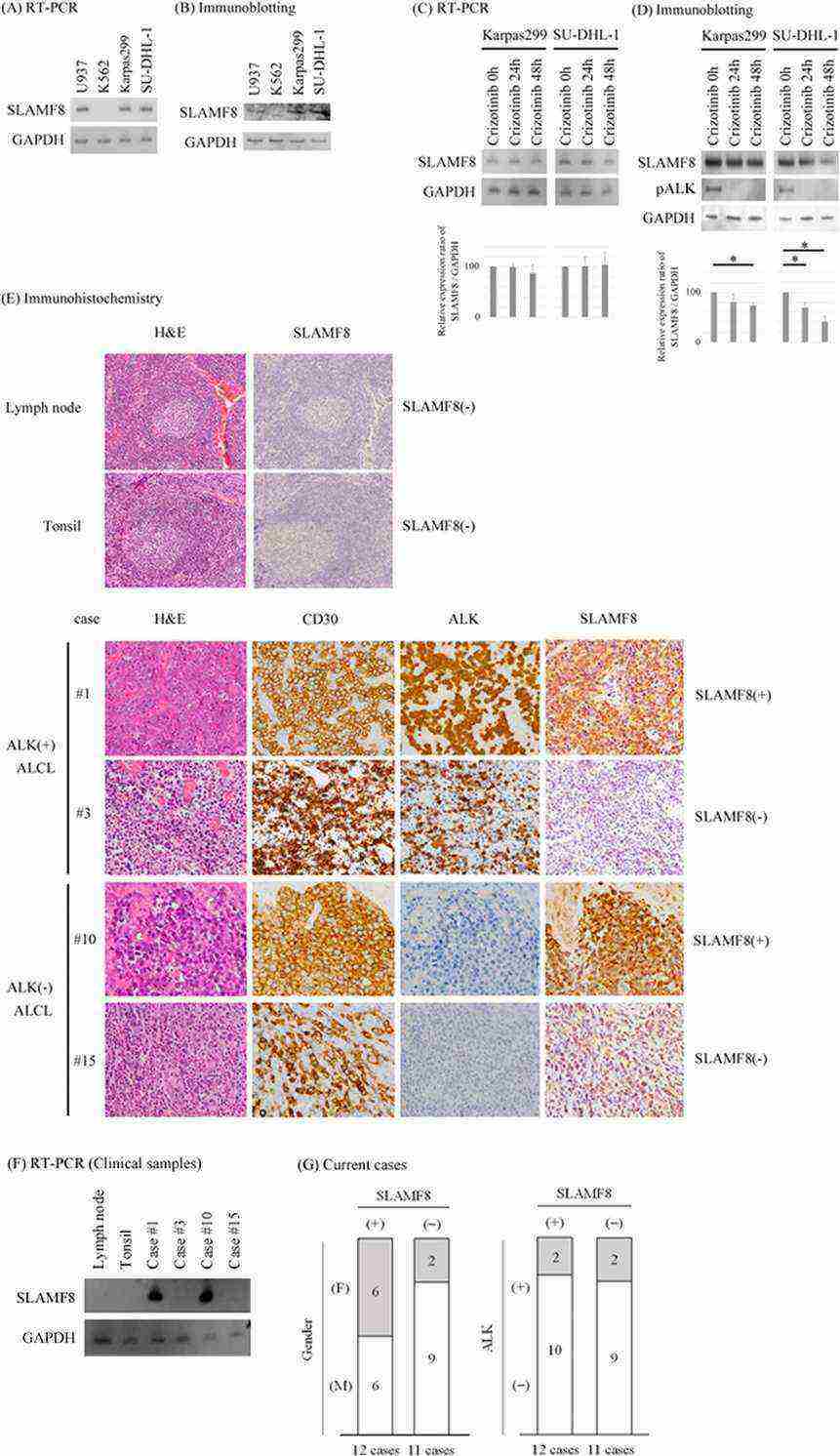 Fig.2 SLAMF8 is expressed in human ALCL cell lines Karpas299 and SU-DHL-1 and in human ALCL pathological samples.
Fig.2 SLAMF8 is expressed in human ALCL cell lines Karpas299 and SU-DHL-1 and in human ALCL pathological samples.References
- Farhangnia P, Ghomi SM, Mollazadehghomi S, Nickho H, Akbarpour M, Delbandi AA. SLAM-family receptors come of age as a potential molecular target in cancer immunotherapy. Front Immunol. 2023;14:1174138.
- Zheng Y, Zhao J, Zhou M, et al. Role of signaling lymphocytic activation molecule family of receptors in the pathogenesis of rheumatoid arthritis: insights and application. Front Pharmacol. 2023;14:1306584.
- Sintes J, Engel P. SLAM (CD150) is a multitasking immunoreceptor: from cosignalling to bacterial recognition. Immunol Cell Biol. 2011;89(2):161-163.
- Gunes M, Rosen ST, Shachar I, Gunes EG. Signaling lymphocytic activation molecule family receptors as potential immune therapeutic targets in solid tumors. Front Immunol. 2024;15:1297473.

