What is PNPLA3 Protein
PNPLA3, or Patatin-Like Phospholipase Domain Containing 3, is a protein that plays a crucial role in lipid metabolism. Its official full name is a mouthful, but its significance in cellular processes cannot be overstated. Also known by the aliases Adiponutrin and Calcium-independent Phospholipase A2-gamma, PNPLA3 belongs to the family of patatin-like phospholipases. These enzymes, characterized by their structural similarity to plant storage proteins called patatins, participate in diverse physiological processes.
Recent research advances have shed light on the intricate details of PNPLA3. Structural studies have revealed its unique features, placing it in the broader context of lipid regulation within cells. Classification of PNPLA3 has evolved as more insights are gained, making it a focal point in understanding metabolic pathways and potential therapeutic interventions.
PNPLA3 Biological Functions and Molecular Mechanisms
The biological functions of PNPLA3 are multifaceted, with a primary role in lipid metabolism. This protein is predominantly expressed in the liver and adipose tissue, underscoring its importance in maintaining metabolic homeostasis. At the molecular level, PNPLA3 is implicated in triglyceride hydrolysis, a key step in lipid catabolism.
Recent studies have identified PNPLA3 as a critical player in lipid droplet dynamics. It regulates the size and composition of lipid droplets, impacting cellular lipid storage and release. The molecular mechanisms involve the enzymatic activity of PNPLA3, which acts as a lipase to break down triglycerides into free fatty acids, influencing lipid homeostasis and energy balance.
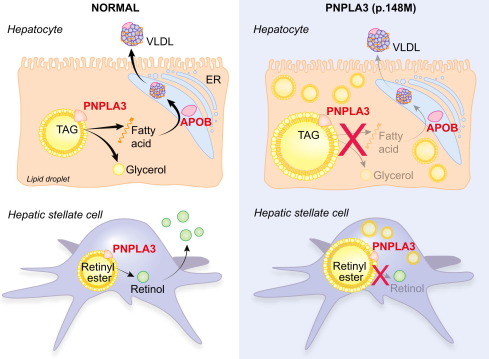
Figure 1. PNPLA3 gene in liver diseases. (Trépo E, et al., 2016)
PNPLA3 Related Signaling Pathway
Understanding the signal pathways associated with PNPLA3 is crucial for unraveling its intricate role in cellular processes. PNPLA3 is intricately linked to the peroxisome proliferator-activated receptor (PPAR) pathway, a signaling cascade involved in lipid metabolism and energy homeostasis. PPARs modulate the expression of genes involved in lipid and glucose metabolism, with PNPLA3 emerging as a downstream effector in this regulatory network.
Elucidating the PNPLA3-related signal pathway provides valuable insights into the broader regulatory mechanisms governing lipid metabolism and energy balance within cells.
PNPLA3 Related Diseases
The dysregulation of PNPLA3 has been associated with various metabolic disorders, making it a target of interest in medical research. One notable connection is with non-alcoholic fatty liver disease (NAFLD), a prevalent condition characterized by excessive fat accumulation in the liver. Genetic variations in the PNPLA3 gene have been identified as risk factors for developing NAFLD, emphasizing the protein's role in liver health.
Additionally, studies have explored links between PNPLA3 variants and other metabolic conditions, including obesity and insulin resistance. The intricate interplay between PNPLA3 and metabolic diseases highlights its potential as a therapeutic target for managing these health challenges.
PNPLA3's Applications in Biomedicine
Beyond its physiological functions, PNPLA3 has garnered attention for its potential applications in biomedical research. The protein's unique role in lipid metabolism makes it a promising target for diagnostic development. Researchers are exploring its utility as a biomarker for identifying individuals at risk of metabolic disorders, allowing for early intervention and personalized treatment strategies.
In the realm of vaccine development, understanding the immunological aspects of PNPLA3 could pave the way for innovative approaches in preventing or managing metabolic diseases. Harnessing the protein's role in cellular processes may offer new avenues for vaccine design targeting metabolic disorders.
In therapeutics, targeting PNPLA3 holds promise for developing drugs that modulate lipid metabolism, providing novel treatment options for conditions such as NAFLD and obesity. By honing in on the molecular mechanisms of PNPLA3, researchers aim to develop pharmaceutical interventions that restore lipid homeostasis and mitigate the impact of metabolic disorders.
Recommended Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| PNPLA3-26HFL | Recombinant Full Length Human PNPLA3 Protein, C-Flag-tagged | Human | Mammalian cells | Flag |
| PNPLA3-367H | Recombinant Human PNPLA3 protein, MYC/DDK-tagged | Human | HEK293 | Myc/DDK |
| PNPLA3-1818H | Recombinant Human PNPLA3, GST-tagged | Human | E.coli | GST |
| PNPLA3-1819H | Recombinant Human PNPLA3 protein, GST-tagged | Human | E.coli | GST |
| PNPLA3-26H | Recombinant Human PNPLA3 Protein, GST-tagged | Human | Wheat Germ | GST |
| PNPLA3-1725H | Recombinant Human PNPLA3 Protein, His (Fc)-Avi-tagged | Human | HEK293 | His (Fc)-Avi |
| PNPLA3-091H | Recombinant Human PNPLA3 Protein, MYC/DDK-tagged, C13 and N15-labeled | Human | HEK293 | C-Myc/DDK |
| PNPLA3-1820H | Recombinant Human PNPLA3 protein, His-tagged | Human | E.coli | His |
| PNPLA3-922HF | Recombinant Full Length Human PNPLA3 Protein, GST-tagged | Human | In Vitro Cell Free System | GST |
| PNPLA3-27H | Recombinant Human PNPLA3 Protein (I148M Mutation), GST tagged | Human | Wheat germ cell free in vitro | GST |
Reference
- Trépo E, et al. PNPLA3 gene in liver diseases. Journal of Hepatology. 2016, 65(2): 399-412.

