Principle and Protocol of Purification of GST Fusion Protein
Glutathione S transfer (GST) can combine with solidified glutathione, and the GST fusion protein is purified by affinity chromatography.
To understand the basic principle of fusion protein expression and purification. Master the expression and purification methods of glutathione S-transferase (GST) fusion protein, as well as the main operation steps and precautions.
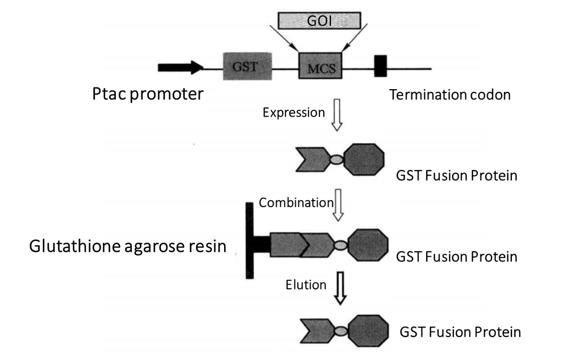 Figure 3-2-1 GST Fusion Protein Purification Process
Figure 3-2-1 GST Fusion Protein Purification Process
pGEX series vectors are used to express glutathione S transferase (GST) fusion protein. Each vector of pGEX series has an open coding frame encoding glutathione S-transferase (GST), followed by a polyclonal site and a stop codon. Lac I gene encodes lac I repressor protein, which binds to the Ptac promoter to repress the expression of GST fusion protein. After IPTG induction, the GST fusion protein is expressed by the disinhibitory effect. The foreign proteins expressed by pGEX series of vectors are fused with GST, so they can be purified by the agarose affinity chromatography resin solidified with glutathione. Because GST has a very high affinity for the substrate, the GST fusion protein can be purified by affinity resin. Finally, the combined GST fusion protein can be eluted with a buffer containing free glutathione. Heart.
1. Main Instruments and Equipment
Pipette, micro centrifuge, 1.5mL and 50mL centrifuge tubes, ice maker, thermostatic shaker, ultra clean workbench, thermostatic incubator, oscillator, centrifuge, metal bath, chromatographic column, ultrasonic crusher, centrifugal filter tube (10000).
2. Experimental Materials
Vector: pGEX-KG
E. coli strain: BL21
3. Main Reagents
(1) LB liquid medium: containing 50 μg/mL ampicillin
(2) 0.1mol/L IPTG
(3) Triton X-100
(4) Glutathione agarose resin
(5) SDS-PAGE gel related reagents
(6) PBS buffer (pH7.4)
| 137 mmol/L | NaCl |
| 2. 7 mmol/L | KCl |
| 10 mmol/L | Na2HPO4 |
| 2 mmol/L | KH2PO4 |
(7) Glutathione elution buffer
| 10 mmol/L | Reduced glutathione |
| 50 mmol/L | Tris-Cl (pH8.0) |
1. Extended Cultivation
(1) After coomassie brilliant blue staining or immunoblotting, the expression of the target protein was determined, and the remaining bacterial fluid was expanded for culture.
(2) Take 1mL of bacterial solution and put it into 100mL LB medium containing ampicillin*1, and shake it in a 1L shake flask at 37°C for more than 2h until it reaches the middle logarithmic growth (A600=0.5-0.6).
(3) The target protein was induced to express at the optimal IPTG concentration, time and temperature determined by the pre-experiment.
(4) After induction for an appropriate time, the cells were collected by centrifugation at 4°C with 5000g for 15min.
2. Preparation Of Crude Extract of Bacterial Protein
(1) Cell precipitation of 100 mL cell culture was suspended in 5 mLPBS buffer.
(2) Add Triton X-100, the final concentration is 1%, and put it on ice.
(3) Crush the bacteria*2 according to the instructions by ultrasonic crusher.

(4) Put the centrifuge tube into the freezing centrifuge, adjust the temperature to 4°C, 12000g, centrifuge for 10min, and remove the cell fragments.
(5) Transfer the crude extract of the supernatant bacterial protein into a new tube.
3. Protein Purification and Detection
(1) Rinse the chromatography column 3 times with 2 mL of deionized water, and equilibrate the column 3 times with 2 mL of LBS buffer.
(2) Invert and mix the glutathione-agarose resin, and take 100 μL into the chromatography column.
(3) Resuspend the agarose with 2mL PBS buffer 3 times.
(4) The supernatant after centrifugation was added to the chromatography column and combined with shaking for 30 min.
(5) Rinse the glutathione-agarose resin 3 times with 600 μL PBS buffer, and collect the effluent.
(6) The protein was eluted 3 times with 250 μL 10 mmol/L reduced glutathione, and the effluent was collected.
(7) Coomassie brilliant blue staining (Figure 3-2-2) or immunoblot detection.
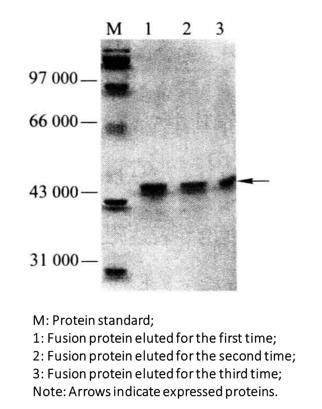 Figure 3-2-2 GST Fusion Protein Purification
Figure 3-2-2 GST Fusion Protein Purification
4. Protein Filtration and Purification
(1) Add 2 mL of purified protein to the centrifuge filter tube, not exceeding the maximum capacity of the tube.
(2) Centrifuge at 5000g for 5 min at 4°C. Repeat several times until there is approximately 500 μL of liquid in the centrifuge tube.
(3) Add 2mLPBS buffer or other buffer to the centrifuge tube and centrifuge at 5000g for 5min at 4°C. Repeat (3) 3 times until there is approximately 500μL of liquid in the centrifuge tube.
(4) Repeat (3) 3 times until there is approximately 200 - 300 μL of liquid in the centrifuge tube*3.
(5) Uncap the centrifuge tube and transfer the liquid from the tube to a new 1.5 mL tube using a pipette.
(6) Detected by Coomassie brilliant blue staining or determined by protein concentration.
1. Cell crushing
Lysozyme or ultrasonic wave can be used to break the bacteria. The method using lysozyme has good repeatability and will not cause severe cell lysis; Ultrasound fragmentation is more efficient. If the stability of the fusion protein is good, ultrasonic fragmentation is recommended. In addition, if ultrasonic breaking method is used, the nucleic acid will also be fragmented when the cell is broken, so the nucleic acid can be prevented from mixing into the recovery solution.
2. Relative molecular weight of GST fusion protein
The relative molecular weight of GST is 26000, so the relative molecular weight of the induced target fusion protein is the sum of the relative molecular weights of GST protein and target protein.
*1 Generally, the amount of protein obtained by using 100mL medium for expanded culture can meet the requirements of other experiments. If the level of protein expression is low, a large amount of expanded culture can be conducted.
*2 If there is no ultrasonic crusher, bacteria can be broken by lysozyme.
*3 The volume of protein is added according to the requirements of the experiment and can be enriched at different concentrations.

