CHIKV Proteins
🧪 Capsid-426V
Source: E.coli
Species: Chikungunya Virus
Tag: His
Conjugation:
Protein Length:

🧪 E2-427V
Source: E.coli
Species: Chikungunya Virus
Tag: His
Conjugation:
Protein Length: 339-692 a.a.

🧪 CHIKVgp2-25C
Source: Insect Cells
Species: Chikungunya
Tag: Non
Conjugation:
Protein Length: amino acids 1-415

🧪 CHIKVgp2-27C
Source: Insect Cells
Species: Chikungunya
Tag: Non
Conjugation:
Protein Length: amino acids 1-415

🧪 CHIKVE1-823C
Source: Insect Cells
Species: Chikungunya virus
Tag: Non
Conjugation:
Protein Length:

🧪 CHIKVE1-727C
Source: Insect Cells
Species: Chikungunya
Tag: Non
Conjugation:
Protein Length:

🧪 E2-336C
Source: Insect Cells
Species: CHIKV
Tag: His
Conjugation:
Protein Length: Ser326-Gln666

🧪 nsp4-4619C
Source: E.coli
Species: CHIKV
Tag: His
Conjugation:
Protein Length: 2228-2474aa

🧪 CHIKV-2767P
Source: HEK293
Species: Pan-species
Tag: His
Conjugation:
Protein Length: Tyr810-His1248
CHIKV Background
Chikungunya virus (CHIKV) is a re-emerging mosquito-borne alphavirus that has evolved from an obscure tropical pathogen into a global public-health threat. Between 2004 and 2023, more than 100 countries reported autochthonous transmission, and millions of cases have been documented (WHO, 2023). The disease burden ranges from explosive outbreaks in naive populations to persistent arthralgia that can last for months or years. This resource page synthesises current scientific knowledge on CHIKV epidemiology, virology, clinical presentation, diagnostics, therapeutics and prevention.
Fig1. Chikungunya virus - CHIKV
What is CHIKV?
Taxonomy and Morphology
CHIKV belongs to the genus Alphavirus within the family Togaviridae. First identified in 1952 in the Makonde Plateau (Tanzania-Mozambique border), its name derives from the Makonde term "kungunyala," meaning "to become contorted," describing patients' stooped posture due to severe joint pain.
The virion is an enveloped, spherical particle 65-70 nm in diameter. The nucleocapsid contains a single-stranded, positive-sense RNA genome of ≈11.8 kb. The genome encodes four non-structural proteins (nsP1-nsP4) and three major structural proteins: the capsid (C), envelope glycoproteins E1 and E2, and a small 6K peptide.
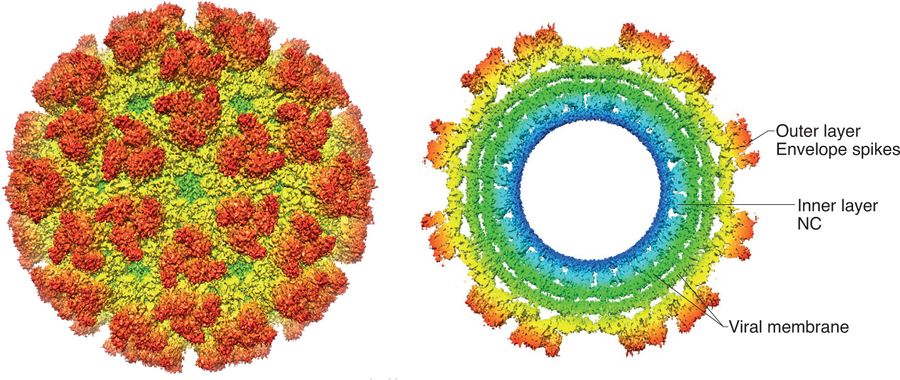
Fig. 2: Chikungunya virus structure. Fox, J. M., & Pierson, T. C. (2022)
Genotypes and Evolution
Three main genotypes are recognized:
Asian - historically restricted to Asia;
East/Central/South African (ECSA) - responsible for the 2004 Indian Ocean outbreak;
West African - limited to West Africa.
A fourth lineage, the Indian Ocean Lineage (IOL), descended from ECSA, acquired an A226V mutation in E1 that improved fitness in Aedes albopictus.
Transmission Cycle
Urban cycle : Aedes aegypti and Ae. albopictus mosquitoes feed on viraemic humans. Mosquitoes acquire the virus by feeding on viremic hosts (human or animal). After a 2-10-day extrinsic incubation period, the virus replicates in salivary glands, enabling transmission to new hosts during subsequent blood meals .
Sylvatic cycle : non-human primates and forest-dwelling Aedes spp. serve as reservoirs in Africa. Trans-ovarial and venereal transmission in mosquitoes contribute to viral persistence during inter-epidemic periods.
Rare non-vector routes include maternal-foetal, blood transfusion and laboratory exposure.
Symptoms of CHIKV Infection
Acute Phase (0-21 days)
- Abrupt high-grade fever (>39 °C)
- Severe, often bilateral poly-arthralgia/arthritis (ankles, wrists, small joints)
- Maculopapular rash (trunk, limbs, palms, soles)
- Myalgia, headache, photophobia, retro-orbital pain, nausea
- Haemorrhagic manifestations are uncommon (<5 %) but may occur in patients with underlying dengue co-infection.
Post-acute & Chronic Phase (>3 weeks)
- Recurrent or persistent joint stiffness and pain in 30-60 % of cases
- Fatigue, depression, Raynaud-like symptoms
- Chronic rheumatological conditions reported: rheumatoid-like arthritis, tenosynovitis, enthesopathy.
Atypical & Severe Manifestations
- Neonates (vertical transmission) : encephalopathy, seizures, limb oedema, myocarditis.
- Adults >65 years or with comorbidities : encephalitis, Guillain-Barré syndrome, acute hepatitis, bullous skin lesions, multi-organ failure.
- Case-fatality rate is low (≤0.1 %), but excess all-cause mortality has been observed during large outbreaks.
How Serious is Chikungunya?
Chikungunya is a disease with considerable epidemic potential. In populations that have never been exposed, attack rates can surpass 50 percent. The burden does not end with the acute phase; up to half of all patients still report a diminished quality of life three years after infection. Outbreaks also carry a heavy economic toll-PAHO calculated that the 2014 Caribbean epidemic generated direct medical expenses and productivity losses of roughly US$ 1.2 billion. Health systems are further strained because chikungunya often circulates alongside dengue and Zika, complicating both diagnosis and clinical management. Finally, certain groups face heightened risk. Neonates, the elderly, and individuals with diabetes or cardiovascular disease are more likely to develop severe forms of the disease.
Mechanisms of CHIKV Infection
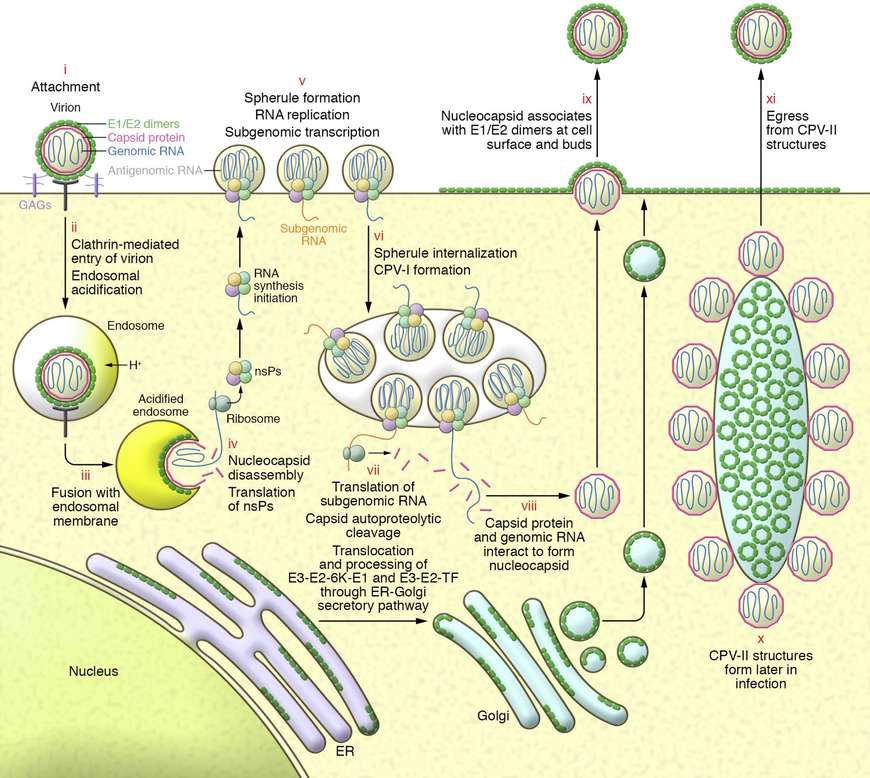
Fig 3. CHIKV replication cycle in mammalian cells. (Laurie A. Silva, Terence S. Dermody, 2017)
Viral Entry
E2 glycoprotein binds to cell-surface receptors including Mxra8 , prohibitin , and ATP synthase β-subunit. Low-pH-triggered conformational changes expose the E1 fusion peptide, facilitating endosomal membrane fusion.
Replication and Assembly
Genomic RNA is translated into the P1, 2, 3, 4 polyprotein, processed into individual nsPs that form the replication complex on modified endosomal membranes (spherules). Negative-strand intermediates serve as templates for 26S sub-genomic RNA encoding the structural proteins. New virions bud from the plasma membrane via interaction of the nucleocapsid with E2/E1 heterodimers.
Host Immune Evasion
- nsP2 degrades STAT1 / 2 , blocking type-I IFN signalling.
- nsP3 interacts with G3BP to inhibit stress-granule formation.
- E2 glycan shielding reduces neutralising antibody binding.
Despite these mechanisms, robust innate and adaptive responses usually clear the virus within 7-10 days.
Pathogenesis of Joint Disease
Virus persists in synovial macrophages, fibroblasts and muscle satellite cells. Pro-inflammatory cytokines ( IL-6 , TNF-α , MCP-1 ) recruit immune cells, leading to synovial hyperplasia, cartilage damage and chronic pain. Auto-antibodies against C1q and citrullinated peptides have been implicated in prolonged rheumatological manifestations.
Diagnostic Methods for CHIKV
Fig4. Diagnostic Methods for CHIKV
Clinical and Epidemiological Criteria
Suspect in any febrile traveller returning from an endemic area within 14 days, particularly if accompanied by severe arthralgia.
Laboratory Confirmation
a) Nucleic acid amplification tests (NAAT)**
Real-time RT-PCR targeting E1, nsP1 or E2 genes; sensitivity 98 % in first 5 days.
RT-LAMP and CRISPR-based assays for resource-limited settings.
b) Serology
IgM-capture ELISA : detectable from day 3-5 and persists 3-6 months.
IgG ELISA : confirms recent or past infection; useful for sero-surveys.
Plaque-reduction neutralisation test (PRNT) : gold standard for distinguishing serological cross-reactivity with other alphaviruses.
c) Antigen detection
NS1-based rapid diagnostic tests (RDTs) under evaluation; limited sensitivity.
d) Point-of-care platforms
Lateral-flow assays using monoclonal antibodies against E2 or capsid proteins.
Smartphone-interfaced biosensors detecting E1 protein in saliva.
Sample Types
Serum, plasma, whole-blood spots, cerebrospinal fluid (CNS disease), cord blood (congenital infection), and dried mosquito pools for entomological surveillance.
Table1. Diagnostic Workflow for Suspected CHIKV
| Time Post-Symptom Onset | Preferred Test | Advantages/Limitations |
|---|---|---|
| 1-7 days | RT-PCR | High sensitivity for acute infection |
| >7 days | IgM/IgG ELISA | Detects immune response; cross-reactivity with other alphaviruses |
| Convalescent Phase | Paired serology (IgG rise) | Confirms recent infection |
Proteins Used in CHIKV Diagnostics
High-quality recombinant proteins are critical for assay development and validation. Creative BioMart provides an extensive catalogue above.
Recombinant E1 glycoprotein - used as capture antigen in IgM/IgG ELISA and lateral-flow strips.
| Cat.No. | Product Name |
|---|---|
| E1-03C | Recombinant Chikungunya mutant Envelope E1 Protein |
| E1-04C | Recombinant Chikungunya wild type Envelope E1 Protein |
| CHI-053 | Recombinant Chikungunya Virus E1 Envelope Protein, His-tagged |
| CHI-054 | Recombinant CHIKV E1 Envelope Protein |
| E1-632C | Recombinant CHIKV E1 Envelope Protein |
| CHI-058 | Recombinant CHIKV Mutant (A226V) E1 Envelope Protein |
| CHIKVE1-823C | Recombinant Chikungunya Wild Type CHIKV E1 Protein |
| CHIKVE1-823C | Recombinant Chikungunya Wild Type CHIKV E1 Protein |
| CHI-051 | Recombinant CHIKV Env 1 Antigen |
| CHI-050 | Recombinant CHIKV-WT-Env 1 Antigen |
Recombinant E2 glycoprotein - high specificity for neutralising antibody detection.
| Cat.No. | Product Name |
|---|---|
| CHI-055 | Recombinant Chikungunya Virus E2 Envelope Protein, hFc-tagged |
| CHI-056 | Recombinant Chikungunya Virus E2 Envelope Protein,, mFc-tagged |
| CHIKVgp2-26C | Recombinant Chikungunya CHIKVgp2 protein |
| CHIKVgp2-27C | Recombinant Chikungunya CHIKVgp2 protein |
| CHIKVgp2-25C | Recombinant Chikungunya CHIKVgp2 protein |
| E2-427V | Recombinant Chikungunya Virus E2 Protein, His-tagged |
| CHI-052 | Recombinant CHIKV Env 2 Antigen |
| E2-336C | Recombinant CHIKV E2/Envelope 2 Protein, His-tagged |
| E2-2427C | Recombinant CHIKV(strain SL-CK1) E2 protein(Ser326-Gln666), His-tagged |
Recombinant Capsid protein - target for NS1 co-detection assays.
| Cat.No. | Product Name |
|---|---|
| Capsid-426V | Recombinant Chikungunya Virus Capsid Protein, His-tagged |
Recombinant nsP1, nsP3 and nsP4 fragments - positive controls in RT-PCR and Western blot.
| Cat.No. | Product Name |
|---|---|
| nsp4-4619C | Recombinant CHIKV nsp4 protein, His-tagged |
CHIKV VLPs (virus-like particles) - safe, non-infectious antigens for vaccine and serology studies.
| Cat.No. | Product Name |
|---|---|
| CHI-059 | Recombinant CHIKV VLP Protein |
| VLP-633C | Recombinant CHIKV VLP Envelope Protein |
All proteins are expressed in HEK293, insect or E. coli systems, with endotoxin levels <0.1 EU/µg.
Treatments for CHIKV Infection
Supportive Care
- Antipyretics: paracetamol (avoid aspirin and NSAIDs until dengue is ruled out).
- Hydration, rest, cold compresses for joint pain.
- Physiotherapy to prevent joint contractures.
Pharmacological Interventions (off-label)
Chloroquine - small RCTs show no benefit in acute viraemia; may relieve chronic arthralgia.
Corticosteroids - short courses (prednisolone 0.5 mg/kg) for refractory joint inflammation.
Disease-modifying anti-rheumatic drugs (DMARDs) - methotrexate, sulfasalazine for persistent rheumatoid-like arthritis.
Monoclonal antibodies - experimental CHIKV-24 (anti-E2) and CHK-166 (broadly neutralising) in phase-I trials.
Investigational Antivirals
Favipiravir - RNA polymerase inhibitor; EC₅₀ ≈ 0.8 µM in cell culture; phase-II efficacy trial in Brazil (NCT04450720).
Ribavirin - variable results; combination with IFN-α under study.
Sofosbuvir and Molnupiravir - repurposed nucleoside analogues with in-vitro activity.
Host-targeting agents - imatinib (ABL kinase inhibitor) and chlorpromazine (inhibitor of endosomal acidification) reduce viral entry.
Convalescent Plasma & IVIG
Case-series suggest reduced viraemia and symptom duration when administered within 5 days of onset; controlled trials ongoing.
Prevention of CHIKV
Vector Control
Environmental : eliminate standing water, cover water-storage containers, manage solid waste.
Chemical : indoor residual spraying, space-spraying of pyrethroids, larvicides (Bti, pyriproxyfen).
Biological : release of Wolbachia-infected Aedes, sterile insect technique, gene-drive approaches.
Personal Protection
- Repellents containing DEET, picaridin, IR3535, or PMD (oil of lemon eucalyptus).
- Long-sleeved clothing, permethrin-impregnated fabrics, insecticide-treated bed nets during daytime naps.
Travel Advice
- CDC and ECDC publish country-specific risk maps.
- Pregnant women should avoid non-essential travel to epidemic areas due to vertical transmission risk.
Drugs and Vaccines for CHIKV
Licensed Vaccines
IXCHIQ® (Valneva, live-attenuated) - single-dose, approved by US FDA November 2023 for adults ≥18 y.
VLA1553 - derived from clone 181/25 with additional attenuating mutations; seroconversion >98 % at 28 days; durable neutralising antibodies for ≥12 months.
Post-marketing surveillance in travellers and military personnel underway.
VIMKUNYA™ (Bavarian Nordic) : Virus-like particle (VLP) vaccine; 97.8% seroconversion rate

Fig5. Drugs for CHIKV
Vaccines in Late-Stage Development
mRNA-1388 (Moderna) - lipid nanoparticle-encapsulated mRNA encoding CHIKV structural proteins; phase-IIb NCT05534345.
MV-CHIK (Themis/BMS) - measles vector expressing CHIKV structural genes; phase-III in the Americas.
Prophylactic Monoclonal Antibodies
CHIKV-24 (AstraZeneca) - half-life extended LALA-PG Fc variant; single IM dose protects for 6 months; phase-III NCT05171647.
VIR-1949 (Vir/GSK) - engineered Fc for enhanced effector function; IND filed 2024.
Pipeline Small-Molecule Drugs
APO866 (NAMPT inhibitor) - inhibits CHIKV replication in vivo mouse models.
BCX4430 (Galidesivir) - broad-spectrum nucleoside analogue active against CHIKV and other RNA viruses; phase-I completed.
Geographic Distribution of CHIKV
Historical Timeline
- 1952-1980s: sporadic outbreaks in Africa and Asia.
- 2004: ECSA genotype spread from Kenya to Indian Ocean islands, India, Southeast Asia.
- 2013: First autochthonous transmission in the Americas (St-Martin); within 2 years >2.9 million suspected cases in 46 countries.
- 2018-2023: Outbreaks in Italy, France, Spain (autochthonous via Ae. albopictus); large epidemics in Brazil, Paraguay, Argentina; ongoing transmission in Ethiopia, Sudan, Chad.
-2025: Local transmission in Italy/France.
-2025: Guangdong outbreak (>4,000 cases) linked to Indian Ocean strain
Risk Maps & Climate Change
Global trade networks and rising temperatures are steadily expanding the range of Aedes vectors, setting the stage for chikungunya virus (CHIKV) to reach new regions. Climate projections based on the high-emission RCP 8.5 scenario indicate that, by 2050, environmental conditions favorable for sustained CHIKV transmission could emerge across the southern United States, broad swaths of central Europe, and densely populated areas of China.
Sentinel Surveillance Networks
Comprehensive sentinel surveillance is rapidly becoming the backbone of global chikungunya preparedness. Under WHO's Global Arbovirus Initiative, launched in 2022, chikungunya, dengue and Zika are now monitored through a single integrated network that pools laboratory resources, standardizes diagnostics, and accelerates outbreak response. Parallel genomic sequencing programs-drawing on open repositories such as GenBank and GISAID-continuously chart the virus's evolutionary trajectory, flagging lineage displacements and novel mutations like the E1-K211E variant that laboratory studies link to enhanced viral fitness.
CHIKV vs Dengue vs Zika
Chikungunya (CHIKV), dengue (DENV) and Zika (ZIKV) viruses are transmitted by the same Aedes mosquitoes, yet they differ in epidemiology, clinical impact and public-health priority. Clinically, dengue is best known for its potential to progress to plasma-leakage syndrome and hemorrhagic shock, with case-fatality rates above 1 % in untreated outbreaks. Chikungunya rarely kills, but the acute polyarthralgia it produces is often more intense and prolonged; up to 50 % of survivors still report impaired quality of life three years later. Zika is usually the mildest of the three, yet its association with congenital microcephaly and other neuro-developmental sequelae makes any infection during pregnancy a sentinel event.
Epidemiologic patterns also diverge. Dengue is endemic across much of the tropics, with four co-circulating serotypes that can sequentially infect the same individual and intensify disease risk. Chikungunya exploded from Africa and Asia into the Americas in 2013-2014, triggering attack rates exceeding 50 % in immunologically naive populations; explosive but often shorter-lived epidemics are typical. Zika had circulated silently for decades before its 2015-2017 pan-American surge, with silent transmission amplified by high proportions of asymptomatic cases and sexual as well as vector-borne spread.
Laboratory differentiation is complicated by overlapping symptoms and cross-reactive antibodies. High-throughput multiplex real-time PCR assays now achieve ≥ 97 % sensitivity and ≥ 99 % specificity for all three viruses in a single tube, while genomic sequencing (GenBank, GISAID) tracks lineage displacement and emerging mutations-such as CHIKV's E1-K211E variant linked to enhanced fitness-informing vaccine and diagnostic updates .
Climate-change models (RCP 8.5) predict that by 2050 large parts of the southern United States, central Europe and densely populated provinces in China will become environmentally suitable for sustained transmission of all three viruses, driven by expanding Aedes vectors and global trade. Colombia's recent nationwide joint spatial analysis reveals dengue dominates most municipalities because of long-standing endemicity, while chikungunya and Zika cluster in socially vulnerable or tourist-heavy areas; higher precipitation favors Zika, whereas chikungunya is more sensitive to crowding and vector density.
For health systems, the simultaneous circulation of these arboviruses magnifies strain on diagnostics, blood supplies and antenatal care. Integrated surveillance-such as WHO's 2022 Global Arbovirus Initiative-now merges CHIKV, dengue and Zika reporting to optimize laboratory capacity and outbreak response, ensuring that no virus is managed in isolation.
| Feature | Chikungunya (CHIKV) | Dengue (DENV) | Zika (ZIKV) |
|---|---|---|---|
| Primary Vector(s) | Aedes aegypti, Aedes albopictus | Aedes aegypti, Aedes albopictus | Aedes aegypti, Aedes albopictus |
| Incubation Period | 2-7 days | 3-14 days | 3-14 days |
| Typical Symptoms | High fever, severe poly-arthralgia (often chronic), rash, headache | High fever, retro-orbital pain, myalgia, rash; warning signs for plasma leakage | Mild fever, maculopapular rash, conjunctivitis, arthralgia; often sub-clinical |
| Case-Fatality Rate | < 0.1 % | 1 %-5 % severe cases; up to 20 % untreated shock | < 0.01 % |
| Chronic/Unique Sequelae | Prolonged (> 3 yr) joint pain in up to 50 % | Post-dengue fatigue syndrome | Congenital Zika syndrome (microcephaly, neuro-developmental defects), Guillain-Barré |
| At-Risk Populations | Neonates, elderly, persons with diabetes/CVD | Children, secondary infections, pregnant women | Pregnant women (any trimester), fetuses, sexually active adults |
| Outbreak Pattern | Explosive epidemics in naive populations (> 50 % attack rates) | Endemic with periodic hyper-epidemics; 4 serotypes | Epidemic waves with high asymptomatic rates |
| Laboratory Confirmation | RT-PCR (viremia d1-7), IgM ELISA (cross-reacts with other alphaviruses) | RT-PCR (NS1, viremia d1-7), IgM/IgG ELISA (serotype-specific PRNT) | RT-PCR (serum/urine), IgM ELISA (cross-reacts with dengue; PRNT confirmation) |
| Key Mutation Monitored | E1-K211E (increased fitness) | Envelope domain III mutations linked to serotype displacement | NS1-A188V (enhanced transmission) |
| Climate Projection (2050, RCP 8.5) | Increased suitability: southern USA, central Europe, China | Same regions plus expansion in highland tropics | Similar expansion; sexual transmission decouples from vector limits |
| Control Strategy | Integrated vector management, personal protection, candidate vaccines in Phase III | WHO-approved vaccines (CYD-TDC, TAK-003), vector control | Vector control, condom use, prenatal screening, no licensed vaccine |
Recent CHIKV Infection Reports (2023-2025)
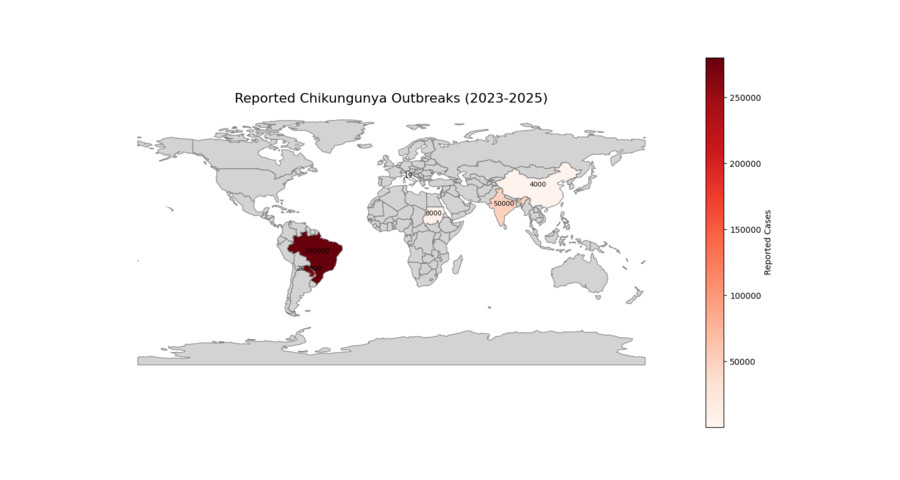
Fig 6. Reported CHIKV Cases Map (2023-2025)
Americas
Brazil, 2023 : >280 000 suspected cases, highest incidence in Minas Gerais and Goiás; ECSA genotype with E1-A226V mutation.
Paraguay, 2023 : 265 000 cases, first national emergency declared since 2020.
Argentina, 2024 : Autochthonous transmission in Buenos Aires province (Ae. albopictus).
Asia-Pacific
India, 2024 : Kerala and Karnataka report >50 000 cases in post-monsoon season; concurrent dengue co-circulation complicates diagnosis.
Thailand, 2023 : Phuket outbreak among European travellers; phylogenetic analysis identifies IOL sub-lineage K210E.
China, 2025 : >4,000 cases (July 2025), with Foshan as epicenter. Weekly cases surged 5-fold to 2,940 by late July
Africa
Senegal, 2023 : Rural outbreak in Kédougou region with spill-over from sylvatic cycle.
Sudan, 2024 : Refugee camps in Darfur report >8 000 cases amid humanitarian crisis and limited vector control.
Europe
Italy, 2023 : Autochthonous cluster in Anzio (Latium); 19 laboratory-confirmed cases linked to Ae. albopictus.
France, 2025 : First sustained transmission in Greater Paris region (Val-de-Marne) during August heatwave.
Travel-related Cases
CDC's GeoSentinel network recorded 612 imported CHIKV cases in US travellers in 2023, primarily from Dominican Republic and India.
ECDC reports 312 imported cases in EU/EEA, with 27 onward autochthonous transmissions.
Resources
References
- Fox, J. M., & Pierson, T. C. (2022). Chikungunya virus assembly and egress. Nature Microbiology, 7(8), 1112-1113. https://doi.org/10.1038/s41564-022-01190-0
- Laurie A. Silva, Terence S. Dermody, Chikungunya virus: epidemiology, replication, disease mechanisms, and prospective intervention strategies. J Clin Invest. 2017;127(3):737-749. https://doi.org/10.1172/JCI84417.
- World Health Organization. Chikungunya Fact Sheet, 2023.
- Centers for Disease Control and Prevention. Yellow Book 2024: Chapter 4 - Chikungunya.
- European Centre for Disease Prevention and Control. Chikungunya: Annual Epidemiological Report 2023.
- Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 2007.
- Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015.








