Hydrophobic Interaction Chromatography
Background
Overview
Hydrophobic Interaction Chromatography (HIC) is a liquid chromatography technique that separates proteins or other biological macromolecules based on their interactions on hydrophobic surfaces. In HIC, the column is coated with a stationary phase with hydrophobic properties. The mobile phase usually contains a concentration of salts that help facilitate the protein's interaction with the hydrophobic stationary phase. As the salt concentration in the mobile phase decreases, the protein will gradually eluate from the column depending on its hydrophobicity. This approach allows separation to be achieved while maintaining the structural integrity of the protein.
Advantages of HIC
Non-denaturable conditions: Hics can be separated under non-denaturable conditions, which means that the three-dimensional structure of proteins or other biological macromolecules is maintained during the separation process, which is particularly important for studying the function and structure of proteins.
Mild separation conditions: Hics are usually performed at low temperatures and neutral pH conditions, which help maintain the protein's biological activity and stability.
High resolution: HIC is capable of efficient separation based on the hydrophobic differences of biomolecules, especially in the separation of structural isomers, aggregates, and degradation products.
Orthogonality: HIC is complementary to other chromatography techniques (such as ion exchange chromatography IEC, size exclusion chromatography SEC, reverse phase chromatography RPC, etc.) and can be used in combination to achieve a more comprehensive separation effect.
Suitable for complex samples: HIC is suitable for processing complex biological samples, such as cell lysate, serum, etc., and can effectively separate target proteins.
Limitations of HIC
Salt dependence: The separation effect of HIC is highly dependent on the type and concentration of salt, which may require optimization for each sample, adding complexity to the operation.
Non-specific: Although Hics have high resolution, their separation mechanism is relatively non-specific and can sometimes lead to co-elution of the target protein with other hydrophobic molecules.
Elution efficiency issues: In some cases, high salt concentrations can lead to irreversible hydrophobic interactions between the protein and the resin, reducing elution efficiency and yield.
Sensitive to operating conditions: HIC is very sensitive to operating conditions such as temperature, pH, buffer concentration, and requires precise control to ensure the separation effect.
Protein conformational change: Although Hics are non-denatational, in some cases, the adsorption of proteins on hydrophobic stationary phases can lead to conformational changes that affect their biological activity.

Stationary Phase Selection
In hydrophobic interaction chromatography (HIC), it is important to select the stationary phase, which should have moderate hydrophobicity, in order to achieve effective separation with different degrees of hydrophobic interaction with solute molecules. The following are some specific requirements for stationary phase selection:
Hydrophobicity: The surface of the stationary phase needs to have weak hydrophobic groups, and the hydrophobicity of these groups is tens to hundreds of times lower than that of the stationary phase used in reverse phase chromatography. Such hydrophobicity is sufficient to interact with hydrophobic groups on protein molecules, but not enough to cause irreversible adsorption and denaturation of proteins.
Non-polarity: The non-polarity of the stationary phase should be weak, which helps to avoid the protein denaturation problems caused by the strong hydrophobicity and organic mobile phase of the stationary phase in reversed-phase chromatography. Mild separation conditions help maintain the natural structure and activity of the protein.
Salt tolerance: Since the mobile phase of HIC is a highly concentrated salt solution, the stationary phase needs to be able to withstand this high-salt environment, keeping its structure stable and function unchanged.
Stability: The stationary phase should exhibit good chemical and physical stability during the experiment to ensure repeatability and reliability.
Compatibility: The fixed response is compatible with the gradient elution technique used and can effectively release adsorbed protein molecules when changing the ionic strength of the mobile phase.
Future Trend
With the development of new technologies, the efficiency and selectivity of Hics are expected to further improve, along with the ability to gently handle biomolecules. The use of HIC technology in combination with other chromatography techniques, such as ion exchange chromatography and affinity chromatography, will provide a more comprehensive solution for the purification of biomolecules. As the demand for personalized treatments increases in the biopharmaceutical industry, HIC technology is likely to evolve in the direction of providing customized solutions. In order to meet the needs of large-scale production, HIC technology will tend to automation and high-throughput processing to improve production efficiency and reduce costs.

Applications
Biopharmaceuticals
Protein purification: HIC is a common method for purifying proteins, especially for its advantages in maintaining protein structure and biological activity. It can be used to purify recombinant proteins, antibodies, enzymes, etc.
Antibody Drug Conjugate (ADC) analysis: HIC can be used to determine the drug antibody ratio (DAR) and drug distribution in the ADC. ADC is a class of targeted therapeutic drugs composed of antibodies, drugs and small molecule linkers. With HIC, the different components in the ADC can be effectively separated and analyzed to evaluate the quality and stability of the drug.
Purification of viral particles: HIC is also suitable for the purification of viral particles because viral particles have specific hydrophobic regions that can be efficiently separated by HIC.
Industrial production
Quality control of bioengineering products: HIC is used in the production process of bioengineering products for purification and quality control of intermediate and final products.
Separation and purification of biological macromolecules: In the production process of biological macromolecules, such as peptides, nucleic acids, HIC can be used as an effective means of separation.
Biological research
Cell membrane protein studies: Since cell membrane proteins are rich in hydrophobic amino acids, HIC can be used to isolate and purify these proteins for structural and functional studies.
Protein-protein interaction studies: Hics can be used to identify and study interactions between proteins, especially when studying the composition and stability of protein complexes.
Case Study
Case Study 1: Aminopropyl-modified Silica Gel
An effective approach in glycoprotein sample preparation involves enriching glycopeptides by solid-phase extraction (SPE) using polar stationary phases in hydrophilic interaction liquid chromatography (HILIC) mode. Different compositions of the elution solvent (acetonitrile, methanol, and isopropanol), along with varying concentrations of elution solvent acidifiers (formic and acetic acid), and different concentrations of acetonitrile for the conditioning and washing solvents (65%, 75%, and 85% acetonitrile) were tested to observe their effects on the glycopeptide enrichment process. Acetonitrile proved the most efficient in enriching glycopeptides. Higher formic acid concentrations in the elution solvent weakened the ionic interactions. Substituting formic acid with acetic acid led to earlier elution of more glycopeptides. The acetonitrile concentration in conditioning and washing solutions played a key role; at 65% acetonitrile, glycopeptides were not retained on the SPE column and were detected in the flow-through fraction. Ultimately, it was proven that the enrichment method was applicable to human plasma samples, resulting in a significant decrease in the abundances of non-glycosylated peptides.
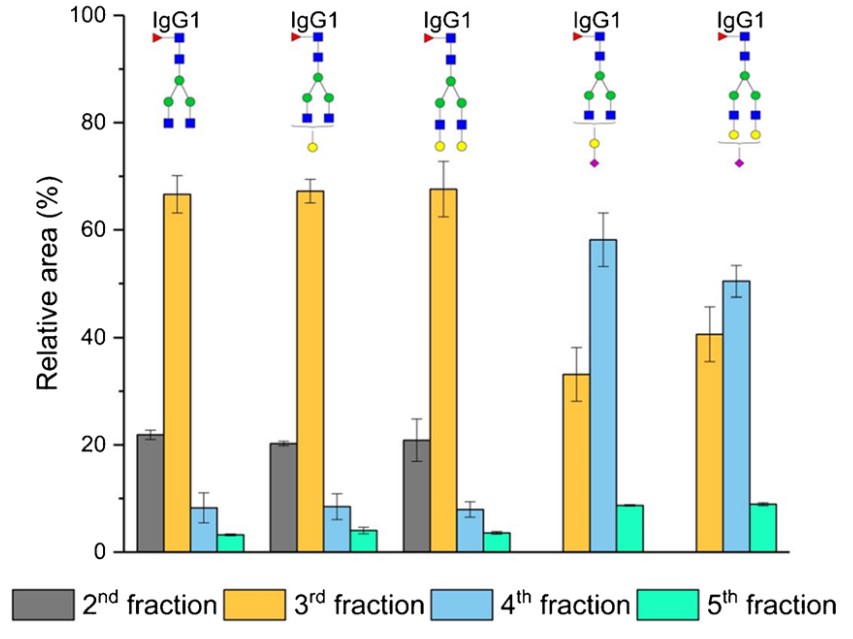
(Katarina Molnarova, 2024)
Fig1. Enrichment of the IgG1 glycopeptides on the aminopropyl-modified silica gel column.
Case Study 2: Phenyl Sepharose 6 Fast Flow
An unusual haloalkaliphilic bacterium known as Halobiforma sp. strain BNMIITR, which was noticed to produce an extracellular alkaline protease, was found in a soil sample from Northern India's Sambhar Lake. On the generation of protease, the effects of dietary elements including nitrogen and carbon sources, amino acids, and growth conditions like temperature and pH were investigated. By solvent precipitation and Hydrophobic interaction chromatography (HIC) on Phenyl Sepharose 6 Fast Flow matrix, the enzyme was purified 31.67 fold. It was determined that the apparent molecular mass was 21 kDa. The pH range where the enzyme was most stable was 6.0-12.0, with a temperature of 50 °C as optimum. When there was alkaline earth metals and heavy metals, protease was discovered to be active. It was evident that the enzyme was a serine type of protease because it was active in the presence of a variety of surfactants, oxidizing and reducing chemicals, and phenylmethylsulfonyl fluoride (PMSF) completely inhibited activity. Enzyme exhibited a wide range of substrate specificity.
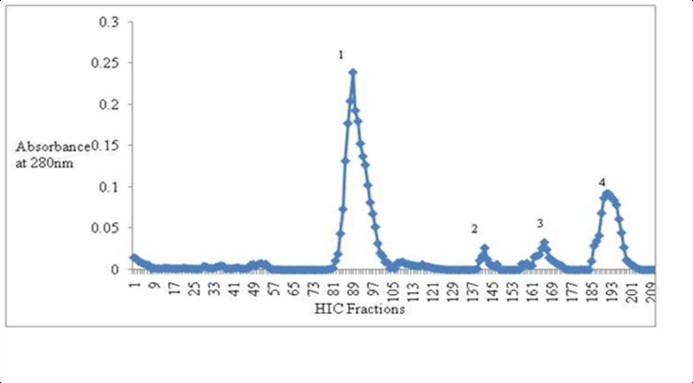
(Meenu Gupta, 2024)
Fig2. Extraction Of extracellular protease from Halobiforma sp. BNMIITR using Phenyl Sepharose 6 Fast Flow column (HIC).
Case Study 3: Butyl, Alkylamide, and Long-Stranded Multialkylamide Ligands
This study presents a comprehensive investigation of the mechanistic understanding of retention and selectivity in hydrophobic interaction chromatography. Retention characteristics have been assessed for three column chemistries, i.e., butyl, alkylamide, and long-stranded multialkylamide ligands, while distinguishing column hydrophobicity and surface area. Salt type and specifically chloride ions proved to be the key driver for improving chromatographic selectivity. The effect was notably more pronounced on the multialkylamide column, as proteins intercalated between the multiamide polymer strands, enabling steric effects. Column coupling proved to be an effective approach for maximizing resolution between molecular variants present in the trastuzumab reference sample and trastuzumab variants induced by forced oxidation. Liquid chromatography-mass spectrometry (LC-MS)/MS peptide mapping experiments after fraction collection indicate that the presence of chloride in the mobile phase enables the selectivity of site-specific deamidation (N30) situated at the heavy chain. Moreover, site-specific oxidation of peptides (M255, W420, and M431) was observed for peptides situated at the Fc region close to the CH2-CH3 interface.
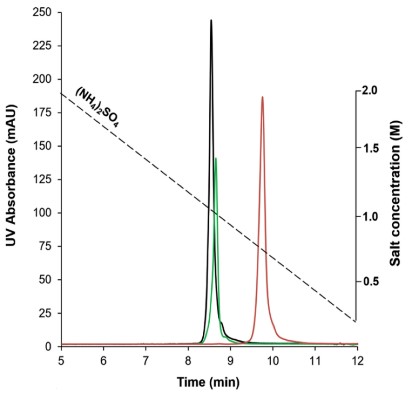
(Raphael Ewonde Ewonde, 2024)
Fig3. Comparison of retention and selectivity obtained on different HIC columns for unstressed trastuzumab, applying inverse gradients of NH4(SO4)2 from 2 to 0 M.
Advantages
- Products Diversity: We offer a wide range of HIC matrix products to meet the needs of different customers and application areas. It includes substrates with different hydrophobicity, particle size, pore size and chemical stability to adapt to the purification requirements of different proteins or biological macromolecules.
- Strong research and development capabilities: We aim to develop a variety of HIC substrates, capable of material synthesis, surface modification and performance testing research and development activities, and constantly innovate and improve products.
- Excellent production technology: We have advanced technology and equipment to produce a wide range of products and ensure batch to batch consistency and product quality.
Creative BioMart can provide various types of IHC products to meet customer’s different research goals. You can also let us know if you have any customized requirements. Please contact us for more product details.
References
- Molnarova K, Chobotova M, Kozlik P. IgG glycopeptide enrichment using hydrophilic interaction chromatography-based solid-phase extraction on an aminopropyl column. Anal Bioanal Chem. 2024;416(8):1867-1881.
- Gupta M, Choudhury B, Navani NK. Production and characterization of an organic solvent activated protease from haloalkaliphilic bacterium Halobiforma sp. strain BNMIITR. Heliyon. 2024;10(3):e25084.
- Ewonde Ewonde R.; et al. Selectivity and Resolving Power of Hydrophobic Interaction Chromatography Targeting the Separation of Monoclonal Antibody Variants. Anal Chem. 2024;96(3):1121-1128.















