Cyclin-Dependent Protein Kinases (CDKs)
Related Symbol Search List
Immunology Background
About Cyclin-Dependent Protein Kinases (CDKs)
Cyclin-dependent protein Kinases (CDKs) are a class of protein kinases that are closely related to the regulation of the cell cycle. They are also involved in many crucial processes, such as cell division and transcription, as well as communication, metabolism, and apoptosis. The kinases are organized in a pathway to ensure that, during cell division, each cell accurately replicates its DNA, and ensures its segregation equally between the two daughter cells. Deregulation of any of the stages of the cell cycle or transcription leads to apoptosis but, if uncorrected, can result in a series of diseases, such as cancer, neurodegenerative diseases (Alzheimer's or Parkinson's disease), and stroke.
CDKs are characterized by needing a separate subunit, a cyclin, that provides domains essential for enzymatic activity. The activity of CDKs is dependent on their regulatory subunits, the cyclins.
The evolutionary expansion of the CDK family in mammals led to the division of CDKs into three cell-cycle-related subfamilies (Cdk1, Cdk4, and Cdk5) and five transcriptional subfamilies (Cdk7, Cdk8, Cdk9, Cdk11, and Cdk20).
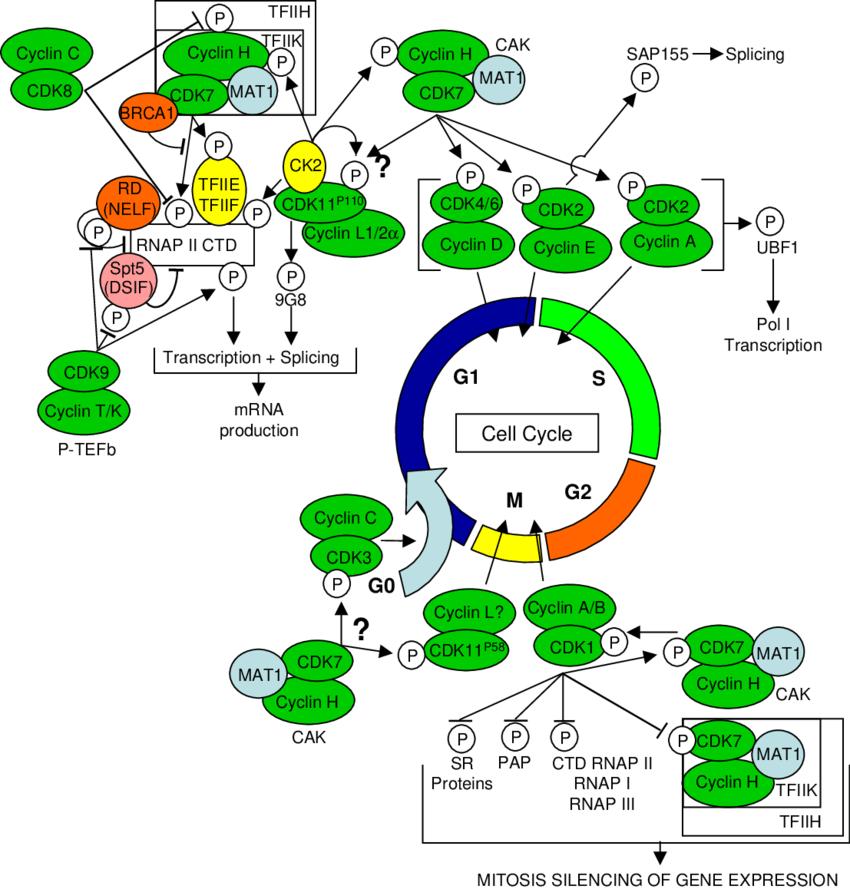 Fig.1 Network of CDK/cyclin complexes regulating cell cycle, transcription and mRNA processing via cross-talk between multiple CDKs. Arrows and bars indicate phosphorylation events resulting in activation and inhibition of downstream protein targets, respectively. (Loyer P, et al., 2005)
Fig.1 Network of CDK/cyclin complexes regulating cell cycle, transcription and mRNA processing via cross-talk between multiple CDKs. Arrows and bars indicate phosphorylation events resulting in activation and inhibition of downstream protein targets, respectively. (Loyer P, et al., 2005)
Mechanism of Action of Cyclin-Dependent Protein Kinases (CDKs)
By definition, a CDK binds a regulatory protein called a cyclin. Without cyclin, CDK has little kinase activity; only the cyclin-CDK complex is an active kinase but its activity can be typically further modulated by phosphorylation and other binding proteins, like p27. CDKs phosphorylate their substrates on serines and threonines, so they are serine-threonine kinases. The consensus sequence for the phosphorylation site in the amino acid sequence of a CDK substrate is [S/T*]PX[K/R], where S/T* is the phosphorylated serine or threonine, P is proline, X is any amino acid, K is lysine, and R is arginine.
CDK levels remain relatively constant throughout the cell cycle and most regulation is post-translational. Most knowledge of CDK structure and function is based on CDKs of S. pombe (Cdc2), S. cerevisiae (CDC28), and vertebrates (CDC2 and CDK2). The major mechanisms of CDK regulation are cyclin binding, CAK phosphorylation, regulatory inhibitory phosphorylation, and binding of CDK inhibitory subunits (CKIs).
- Cyclin Binding
The mechanism of action of CDKs involves their association with cyclins, forming active holoenzymes. These CDK-cyclin complexes phosphorylate target proteins involved in cell cycle progression, gene expression, and other cellular functions. The phosphorylation events mediated by CDKs regulate the activity, localization, and stability of their target proteins, thus coordinating key cellular processes.
- Phosphorylation and Dephosphorylation Events
CDK activity is also regulated by phosphorylation and dephosphorylation events, which can either enhance or inhibit their kinase activity. CDK inhibitors, such as the p21 and p27 proteins, bind to CDK-cyclin complexes and prevent their activation, thus acting as negative regulators of the cell cycle.
Functions of Cyclin-Dependent Protein Kinases (CDKs)
- Cell Cycle Regulation
CDKs are central regulators of the cell cycle, ensuring proper progression through the different phases (G1, S, G2, and M phases). They phosphorylate key substrates involved in cell cycle transitions, allowing the cell to progress from one phase to another.
- Gene Expression Control
CDKs also regulate gene expression by phosphorylating transcription factors and chromatin-associated proteins. These phosphorylation events influence the accessibility of DNA and the recruitment of transcriptional machinery, thereby modulating gene transcription.
- Development and Differentiation
CDKs play critical roles in cell differentiation and development. They control the timing and coordination of cell division during embryogenesis and tissue development, ensuring proper growth and differentiation of cells and tissues.
- Cancer and Therapeutic Targets
Dysregulation of CDK activity is a hallmark of many cancers. Aberrant CDK activity can lead to uncontrolled cell proliferation and tumor formation. Consequently, CDKs have emerged as promising therapeutic targets, and selective CDK inhibitors have been developed for cancer treatment, such as seliciclib and alvocidib. Complications of developing a CDK drug include the fact that many CDKs are not involved in the cell cycle, but in other processes such as transcription, neural physiology, and glucose homeostasis.
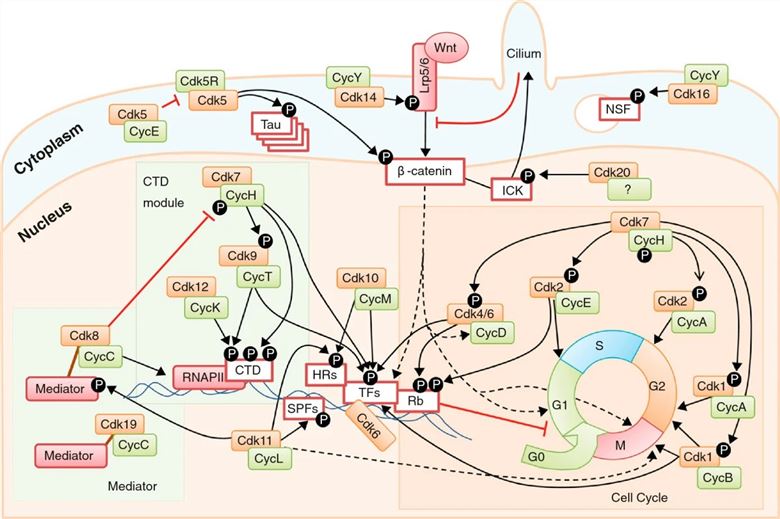 Fig.2 An overview of CDK functions in the cell. (Malumbres M, 2014)
Fig.2 An overview of CDK functions in the cell. (Malumbres M, 2014)
Available Resources for Cyclin-Dependent Protein Kinases (CDKs)
At Creative BioMart, we offer a range of resources to support CDK-related research, including recombinant proteins, cell and tissue lysates, protein pre-coupled magnetic beads, and others. These tools can be used for various applications such as protein expression analysis, activity assays, and protein-protein interaction studies. The following cyclin-dependent protein kinases (CDKs) are displayed, click to view all related molecules/targets and research reagents. Please feel free to contact us with any questions or requests.
References:
- Łukasik P, Załuski M, Gutowska I. Cyclin-Dependent Kinases (CDK) and Their Role in Diseases Development-Review. Int J Mol Sci. 2021;22(6):2935. Published 2021 Mar 13.
- Loyer P, Trembley JH, Katona R, Kidd VJ, Lahti JM. Role of CDK/cyclin complexes in transcription and RNA splicing. Cell Signal. 2005;17(9):1033-1051
- Malumbres M. Cyclin-dependent kinases. Genome Biol. 2014;15(6):122.

