IGF Receptor
Related Symbol Search List
Immunology Background
Available Resources for IGF Receptor Research
Creative BioMart offers powerful support for researchers studying IGF receptors, providing a range of carefully curated products, customizable services, and extensive resources.
- Our offerings include recombinant proteins, pre-coupled magnetic beads, cell and tissue lysates, and more, all designed to meet specific research needs.
- In addition to our product line, we are committed to providing a wealth of information on IGF receptors. Our resources cover essential topics such as involved pathways, protein functions, interacting proteins, related articles, and research areas, serving as a valuable reference for researchers looking to deepen their understanding of the critical role these molecules play in various physiological processes.
Our Featured Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| IGF1R-3148H | Recombinant Human IGF1R, His tagged | Human | Human Cell | His |
| Igf1r-30R | Recombinant Rat Igf1r protein, His-tagged | Rat | E.coli | His |
| NSR-623H | Recombinant Human INSR, His tagged | Human | HEK293 | His |
About IGF Receptors
Insulin-like Growth Factor (IGF) receptors are cell surface receptors that play a crucial role in mediating the biological actions of IGFs. Here's an introduction to IGF receptors:
Insulin-like Growth Factor 1 Receptor (IGF-1R)
- Structure: IGF-1R is a transmembrane receptor composed of two α-subunits and two β-subunits linked by disulfide bonds. The extracellular region of the α-subunit contains the ligand-binding site, while the β-subunit spans the cell membrane and possesses an intracellular tyrosine kinase domain.
- Ligand Binding and Activation: IGF-1R binds specifically to IGF-1 and IGF-2 with high affinity. Upon ligand binding, IGF-1R undergoes autophosphorylation, where the tyrosine kinase domain phosphorylates specific tyrosine residues within the receptor itself, leading to the activation of downstream signaling pathways.
- Downstream Signaling: Activated IGF-1R initiates intracellular signaling cascades that promote cell growth, proliferation, survival, and differentiation. Key signaling pathways include the PI3K-Akt pathway, which regulates cell survival and growth, and the Ras-MAPK pathway, which controls cell proliferation and differentiation.
- Interaction with Insulin Receptor (IR): IGF-1R shares significant structural and functional similarities with the Insulin Receptor (IR). The two receptors can form hybrid receptors, consisting of one IGF-1R α-subunit and one IR α-subunit. These hybrid receptors have distinct ligand-binding properties and signaling characteristics.
Insulin-like Growth Factor 2 Receptor (IGF-2R) / Mannose 6-Phosphate Receptor (M6P Receptor)
- Structure: IGF-2R, also known as M6P receptor, is a type I transmembrane protein. It consists of a large extracellular region that contains multiple binding domains for mannose 6-phosphate residues, a transmembrane domain, and a short cytoplasmic tail.
- Ligand Binding and Function: IGF-2R/M6P receptor binds to IGF-2 and a variety of other ligands modified with mannose 6-phosphate residues. Unlike IGF-1R, IGF-2R does not possess intrinsic kinase activity. Its main function is to internalize and degrade ligands, playing a role in the clearance and degradation of IGF-2 and certain other proteins.
- Regulation of IGF-2 Activity: IGF-2R helps regulate the availability and activity of IGF-2 by sequestering and targeting it for lysosomal degradation. This receptor serves as a "sink" for IGF-2, preventing excessive IGF-2 signaling and maintaining the balance of IGF-2 levels in the body.
Insulin-like Growth Factor Binding Protein-like 1 (IGFLR1)
- Function: IGFLR1, also known as IGFBP-rP1 or MAC25, is a member of the Insulin-like Growth Factor Binding Protein (IGFBP) family. Its precise function is not yet fully understood, but it is believed to interact with IGFs and modulate IGF signaling and bioavailability.
- Expression and Regulation: IGFLR1 is expressed in various tissues, including the liver, kidney, and reproductive organs. Its expression can be regulated by factors such as growth factors, cytokines, and hormones. However, the exact mechanisms and physiological roles of IGFLR1 are still being investigated.
- Potential Functions: IGFLR1 has been implicated in diverse biological processes, including cell proliferation, apoptosis, and tumor development. It may act as a modulator of IGF signaling by interacting with IGFs or other IGFBPs, but further research is needed to fully understand its specific functions and mechanisms of action.
Insulin Receptor (INSR)
- Function: The Insulin Receptor (INSR) is a cell surface receptor that mediates the actions of insulin, a hormone involved in regulating glucose metabolism, cellular uptake of nutrients, and various metabolic processes.
- Structure: INSR is composed of two α-subunits and two β-subunits linked by disulfide bonds. The α-subunit contains the insulin-binding site, while the β-subunit possesses an intracellular tyrosine kinase domain.
- Insulin Signaling: Upon insulin binding, INSR undergoes autophosphorylation, activating its tyrosine kinase domain. This triggers the phosphorylation of downstream signaling molecules, such as IRS, leading to the activation of signaling pathways like PI3K-Akt and Ras-MAPK. Insulin signaling plays a crucial role in glucose uptake, glycogen synthesis, and metabolism regulation.
- Roles in Diabetes and Metabolic Disorders: Dysregulation of INSR signaling is a hallmark of insulin resistance, a condition that underlies type 2 diabetes and metabolic disorders. Impaired INSR signaling can lead to decreased insulin sensitivity and disrupted glucose homeostasis. Understanding INSR function and regulation is essential in managing and treating insulin resistance-related conditions.
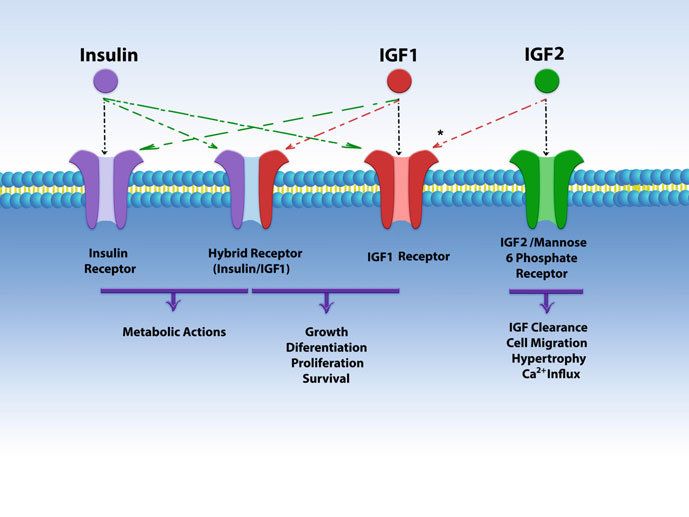 Fig.1 Schematic structure of insulin, IGFs, and their receptors. (Aguirre GA, et al., 2018)
Fig.1 Schematic structure of insulin, IGFs, and their receptors. (Aguirre GA, et al., 2018)
The Key Signaling Pathways of IGF Receptors
The signaling pathways of IGF receptors, particularly the Insulin-like Growth Factor 1 Receptor (IGF-1R), involve multiple interconnected cascades that regulate various cellular processes. The two major signaling pathways associated with IGF receptors are the PI3K-Akt pathway and the Ras-MAPK pathway. Here's an overview of these pathways:
PI3K-Akt Pathway
a. Ligand Binding: When IGF-1 or IGF-2 binds to the IGF-1R, it induces a conformational change in the receptor, leading to autophosphorylation of tyrosine residues on the receptor.
b. Insulin Receptor Substrates (IRS) Activation: Phosphorylated tyrosine residues on IGF-1R serve as docking sites for insulin receptor substrates (IRS), mainly IRS-1 and IRS-2. IRS proteins are then phosphorylated by IGF-1R.
c. PI3K Activation: The phosphorylated IRS proteins recruit and activate phosphatidylinositol 3-kinase (PI3K), which converts phosphatidylinositol bisphosphate (PIP2) to phosphatidylinositol trisphosphate (PIP3).
d. Akt Activation: PIP3 serves as a docking molecule for the serine/threonine kinase Akt (protein kinase B). Akt is recruited to the plasma membrane, where it is phosphorylated and activated by phosphoinositide-dependent kinase-1 (PDK1) and mammalian target of rapamycin complex 2 (mTORC2).
e. Downstream Effects: Activated Akt phosphorylates and regulates various downstream targets involved in cell growth, survival, and metabolism. This includes the activation of mTORC1, which stimulates protein synthesis, and the inhibition of pro-apoptotic factors like Bad and caspase-9, promoting cell survival.
Ras-MAPK Pathway
a. Ligand Binding: IGF ligands binding to IGF-1R trigger the autophosphorylation of tyrosine residues on the receptor.
b. Recruitment of Adaptor Proteins: Phosphorylated tyrosine residues on IGF-1R serve as docking sites for adaptor proteins, such as Grb2 (growth factor receptor-bound protein 2).
c. Activation of Ras: Adaptor proteins, particularly Grb2, recruit and activate guanine nucleotide exchange factors (GEFs) that facilitate the activation of Ras, a small GTPase protein.
d. MAPK Activation: Active Ras triggers a downstream signaling cascade involving the activation of the mitogen-activated protein kinase (MAPK) pathway. This pathway includes sequential activation of Raf, MEK (MAPK/ERK kinase), and ERK (extracellular signal-regulated kinase).
e. Nuclear Translocation and Gene Expression: Activated ERK translocates to the nucleus, where it phosphorylates and activates transcription factors, such as Elk-1, leading to the regulation of gene expression associated with cell growth, differentiation, and survival.
These pathways, the PI3K-Akt pathway, and the Ras-MAPK pathway, are prominent downstream signaling cascades activated by IGF receptors. They converge on several intracellular targets, coordinating cellular responses related to growth, proliferation, differentiation, and survival. The exact outcomes and effects of IGF receptor signaling depend on the specific cellular context and the interplay with other signaling networks.
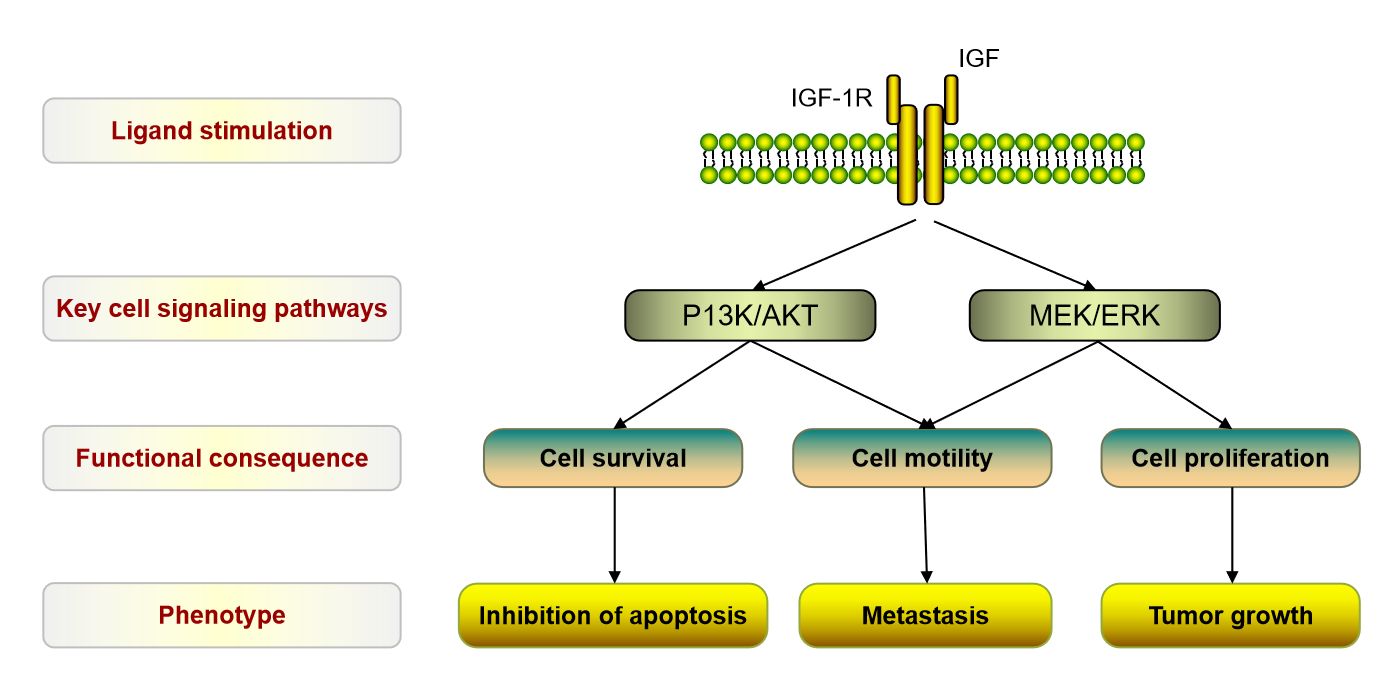 Fig.2 The Key signaling pathways of IGFR1. (Mulvihill M J, et al., 2009)
Fig.2 The Key signaling pathways of IGFR1. (Mulvihill M J, et al., 2009)
If you have any questions, requirements, or cooperation intentions, please feel free to contact us. We very much look forward to working with you and helping you achieve research and commercial success.
Related References
- Aguirre GA, González-Guerra JL, Espinosa L, Castilla-Cortazar I. Insulin-Like Growth Factor 1 in the Cardiovascular System. Rev Physiol Biochem Pharmacol. 2018;175:1-45.
- Mulvihill M J, Cooke A, Rosenfeld-Franklin M, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor[J]. Future medicinal chemistry, 2009, 1(6): 1153-1171.

