Nogo Proteins and Receptors
Related Symbol Search List
Immunology Background
Background
Nogo proteins are pivotal components of the central nervous system (CNS), primarily influencing neural development and regeneration. Their primary role lies in inhibiting axonal growth and neural plasticity, particularly following injury.
Key Features of Nogo Proteins
| Types of Nogo Proteins |
|
| Mechanism of Action |
|
Nogo Receptors
| Nogo-66 Receptor (NgR) |
|
| Co-Receptors | NgR functions alongside various co-receptors, such as:
|
Biological Implications
Nogo proteins and their receptors are critical in maintaining neuronal stability and preventing abnormal growth. However, their inhibitory effects pose challenges for recovery following CNS injuries or neurodegenerative diseases. As a result, they are being studied as potential targets for therapeutic interventions aimed at enhancing axonal regeneration in the CNS.
Functions of Nogo Proteins and Receptors
- Inhibition of Axonal Growth: Nogo proteins, particularly Nogo-A, act as potent inhibitors of axonal growth in the central nervous system. By interacting with their receptors on neurons, they restrict the extension of axons, limiting neural circuit remodeling and plasticity.
- Regulation of Neural Plasticity: Nogo proteins and their receptors play a crucial role in modulating neural plasticity. They help maintain the integrity of existing neural connections and prevent aberrant axonal sprouting, contributing to the stability of neural circuits.
- Axonal Regeneration Inhibition: Following neural injury or in pathological conditions, Nogo proteins exert inhibitory effects on axonal regeneration. This inhibition hampers the regrowth of damaged axons and restricts the potential for neural repair and functional recovery.
- Guidance of Neuronal Migration: Nogo proteins and their receptors are involved in guiding neuronal migration during development. They help regulate the movement of neurons to their appropriate locations within the developing nervous system.
- Regulation of Synaptic Plasticity: Nogo proteins and receptors also influence synaptic plasticity, impacting the strength and efficacy of synaptic connections between neurons. Their actions contribute to the maintenance of stable neural circuits and neuronal communication.
- Mediation of Myelination: Nogo proteins and receptors may play a role in regulating myelination, the process by which axons are wrapped in myelin sheaths. This function is crucial for the efficient transmission of nerve signals and overall neural function.
- Promotion of Neuronal Survival: Certain interactions involving Nogo receptors, such as those with neurotrophic factors, can promote neuronal survival and prevent cell death, contributing to the maintenance of healthy neural populations.
Nogo proteins and receptors play a complex role in neuronal signaling, balancing the need for stability and inhibition of hyperactivity in the CNS environment. Understanding their mechanisms is crucial for developing strategies to promote neural repair and rehabilitation following injury.
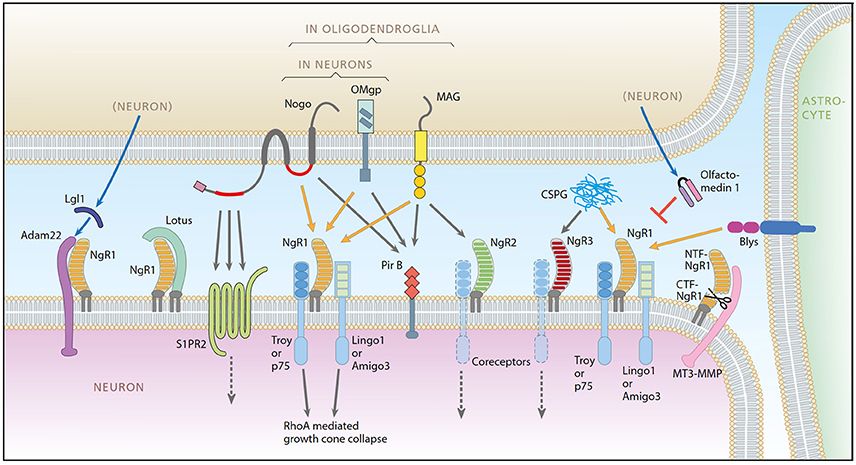 Fig.1 A schematic representation of the Nogo system. (Smedfors G, et al., 2018)
Fig.1 A schematic representation of the Nogo system. (Smedfors G, et al., 2018)Nogo Proteins and Receptors-Related Molecules
The following is an introduction to some Nogo proteins and receptor-related molecules:
| Type | Details |
|---|---|
| Nogo Proteins and Related Molecules |
|
| Ligands and Receptors |
|
| Neurotrophic Factors and Receptors |
|
| Signal Transduction Proteins |
|
| Additional Molecules |
|
These proteins and receptors are interconnected in a complex network that influences neuronal growth, survival, and regeneration in the CNS. Nogo proteins, alongside receptors like Rtn4r, play significant roles in inhibiting axonal regeneration, with various ligands and signaling proteins modulating these processes, providing potential therapeutic targets for CNS injuries and neurodegenerative conditions.
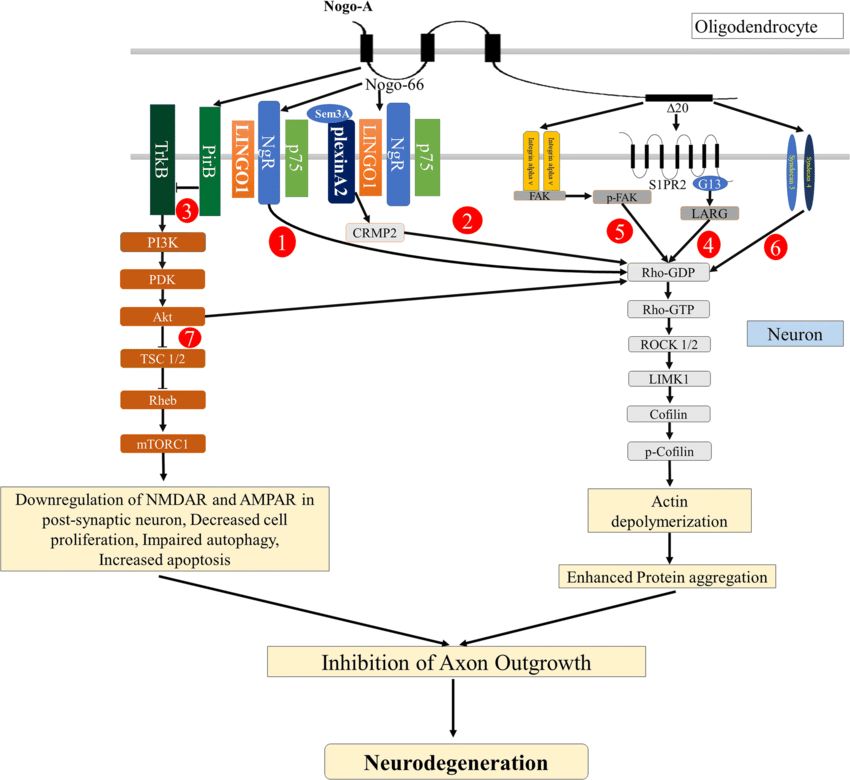 Fig.2 Nogo-A signaling pathways leading to inhibition of axon outgrowth. (Pradhan LK, et al., 2021)
Fig.2 Nogo-A signaling pathways leading to inhibition of axon outgrowth. (Pradhan LK, et al., 2021)The Functions and Roles of Nogo Proteins and Receptors in the Nervous System
Nogo proteins, particularly Nogo-A, play crucial roles in the nervous system, particularly regarding neural development, injury response, and regeneration. Here's a detailed explanation of their functions and roles:
Inhibition of Axonal Regeneration
- CNS vs. PNS: Unlike the peripheral nervous system (PNS), where axonal regeneration can occur effectively, the central nervous system (CNS) is prone to limited regeneration after injury. Nogo-A is a key player in creating this inhibitory environment.
- Growth Cone Collapse: When Nogo proteins bind to their receptors, such as the Nogo-66 receptor (NgR), they activate RhoA and other signaling pathways, leading to the collapse of the growth cone—a structure at the tip of a growing axon. This collapse halts the axon's ability to extend and regenerate.
Regulation of Neuronal Plasticity
- Synaptic Stability: Nogo proteins help maintain the stability of synapses, which is critical for proper neuronal function and communication. They prevent excessive synaptic remodeling, ensuring that established connections remain intact.
- Homeostasis: By controlling synaptic growth and stability, Nogo proteins contribute to homeostasis in neuronal networks, which is necessary for learning and memory processes.
Involvement in Myelination
- Oligodendrocytes: Nogo-A is predominantly expressed in oligodendrocytes, the myelin-forming cells in the CNS. It plays a role in regulating oligodendrocyte development and the formation of myelin sheaths around axons.
- Myelin-Associated Inhibition: Nogo proteins are part of a broader class of myelin-associated inhibitors (which includes other proteins like OMgp and MAG) that can signal to neurons, further contributing to the inhibition of axonal growth in the adult CNS.
Molecular Interactions and Pathways
NgR and Co-Receptors: The Nogo-66 receptor (NgR) initiates signaling cascades upon binding Nogo-A. Importantly, NgR lacks a transmembrane domain, meaning it needs to interact with co-receptors such as:
- p75 Neurotrophin Receptor (p75NTR): Facilitates signal transduction and amplifies the inhibitory signals of Nogo.
- LRP (LDL Receptor-Related Protein): May also interact with NgR to modulate the signaling pathway.
- RhoA Activation: NgR activation leads to RhoA activation, which governs cytoskeletal dynamics, promoting the reorganization of actin filaments and ultimately leading to growth cone collapse.
Role in Neurodegenerative Diseases and Injury Response
- Regenerative Barriers: In conditions like multiple sclerosis, spinal cord injuries, and other neurological disorders, upregulation of Nogo-A can exacerbate the inability of injured CNS axons to regenerate.
- Therapeutic Target: Research is ongoing to explore therapeutic strategies that either block Nogo signaling or mimic its inhibition to enhance regeneration after CNS injuries. This includes the development of antibodies against Nogo-A, as well as small molecules targeting NgR.
Impacts on Neurodevelopment
- Developmental Regulation: Nogo proteins are also involved in the development of the nervous system. They can influence neuronal migration and affect the formation of neural circuits during development.
- Neural Network Establishment: By limiting excessive growth and refining connections, Nogo proteins play a role in shaping functional neural networks.
Nogo proteins, particularly through Nogo-A, serve as critical inhibitors of axonal regeneration and key regulators of neuronal plasticity, synaptic stability, and development within the CNS. Their multifaceted roles make them critical targets for potential therapeutic interventions aimed at enhancing recovery from CNS injuries and addressing neurodegenerative diseases. Understanding their functions can lead to strategies that modulate their effects to promote neuronal repair and functional recovery.
The Role and Potential Applications of Nogo Proteins and Receptors in Neurodegenerative Diseases
Role in Neurodegenerative Diseases
Inhibition of Regeneration
After neuronal damage, such as that seen in diseases like multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS), Nogo proteins inhibit the growth and repair of damaged axons. This contributes to the progression of neurological deficits.
Impact on Plasticity
Nogo proteins limit synaptic plasticity, which is crucial for learning and memory. In conditions like Alzheimer's disease, where synaptic dysfunction is prevalent, the overexpression of Nogo may exacerbate cognitive decline.
Immune Response
In conditions like MS, Nogo proteins may also interact with the immune system, influencing inflammatory responses and the overall neuroinflammatory environment.
Potential Applications
Therapeutic Targeting
Blocking Nogo Pathways: Developing inhibitors or antagonists that block Nogo-A interaction with its receptors (e.g., NgR) could enhance axonal regeneration and functional recovery after neuronal injury.
Gene Therapy: Gene silencing techniques (like RNA interference) targeting Nogo proteins might promote regenerative processes in affected neurons.
Neuroprotection
Compounds that inhibit Nogo signaling could provide neuroprotective effects, preserving neuronal integrity in diseases like Parkinson's and Alzheimer's by supporting the survival of neurons under stress.
Enhancing Cognitive Function
In Alzheimer's disease, modulating Nogo expression may help restore synaptic function and improve cognitive deficits, promoting better outcomes in memory and learning.
Rehabilitation Strategies
Interventions (like physical therapy) combined with Nogo receptor antagonists could synergistically promote recovery after traumatic injuries, potentially benefiting patients with neurodegenerative diseases as well.
Custom Drug Development
Identifying specific small molecules or monoclonal antibodies that target Nogo proteins could lead to tailored treatments for various neurodegenerative conditions, enhancing the effectiveness of current therapies.
Nogo proteins and their receptors are central players in hindering neuronal repair and regeneration in neurodegenerative diseases. By targeting these pathways, there is significant potential to develop new therapeutic strategies aimed at promoting neural regeneration, protecting neuronal health, and improving cognitive functions. Research in this area is ongoing and could lead to breakthroughs in how we treat a variety of neurodegenerative disorders.
Case Study
Case 1: Vaccaro G, Dumoulin A, Zuñiga NR, Bandtlow CE, Stoeckli ET. The Nogo-66 receptors NgR1 and NgR3 are required for commissural Axon pathfinding. J Neurosci. 2022;42(20):4087-4100.
Nogo-66 receptors (NgR1-3) are a class of proteins linked to glycerophosphatidylinositol and belong to a superfamily of leucine-rich repeats. Through binding to myelin-associated inhibitors, NgRs play an important role in the inhibition of axonal regeneration after spinal cord injury. Although their role in limiting synaptic plasticity and axonal growth in the adult central nervous system (CNS) has been described, little is known about their role in neurodevelopment.
In this study, mRNAs for NgR1 and NgR3 were found to be expressed during spinal cord development in chick embryos. In particular, they were detected in the dI1 subpopulation of crossover neurons during the period when neurons navigate along the floor plate. To assess the potential role of NgR1 and NgR3 in axon guidance, the investigators down-regulated both receptors using an in vivo RNA interference technique and analyzed them by tracing the trajectory of axons in an open book-prepared spinal cord. The results showed that loss of either NgR1 or NgR3 caused axons to stall in the midline region and interfered with the cephalad steering of overcrossed axons. In addition, the results show that the function of NgR1 requires the involvement of the neuron PlexinA2, whereas NgR3 does not.
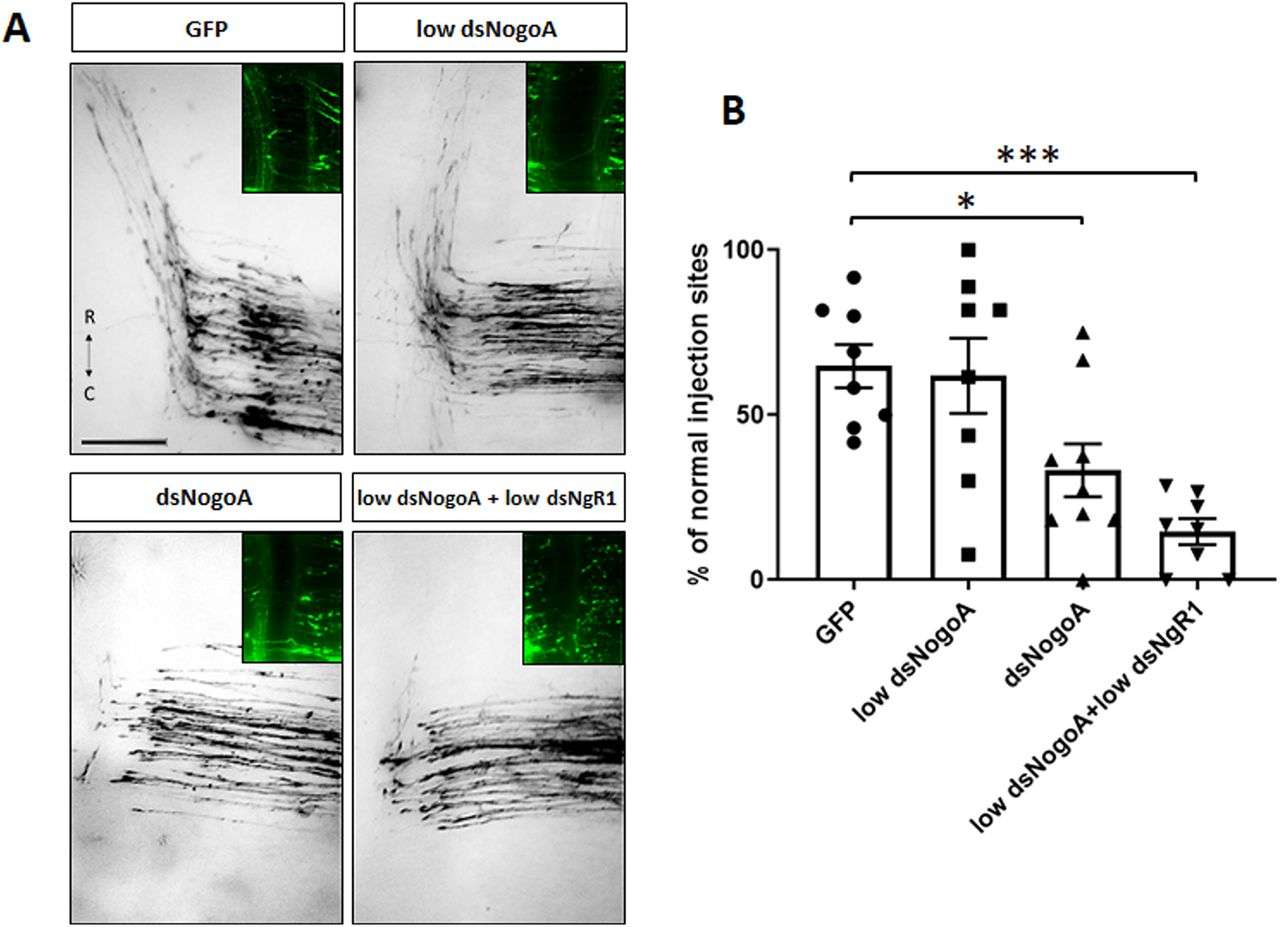 Fig.1 NogoA regulates commissural axon guidance.
Fig.1 NogoA regulates commissural axon guidance.Case 2: Thomas RA, Gibon J, Chen CXQ, et al. The Nogo receptor ligand LGI1 regulates synapse number and synaptic activity in hippocampal and cortical neurons. eNeuro. 2018;5(4):ENEURO.0185-18.2018.
Leucine-rich glioma-inactivated protein 1 (LGI1) is a secreted neuronal protein that functions as a ligand for Nogo receptor 1 (NgR1). Mutations in LGI1 lead to autosomal dominant lateral temporal lobe epilepsy in humans, while complete deletion of LGI1 in mice results in severe epileptic seizures causing early postnatal death. NgR1, crucial for central nervous system synapse and circuit development, restricts plasticity in the adult cortex through RhoA activation.
These connections prompted an investigation into LGI1's impact on synapse formation both in vitro and in vivo. Findings reveal that LGI1 application enhances synaptic density in neuronal cultures and that LGI1-deficient hippocampi exhibit fewer dendritic mushroom spines compared to wild-type (WT) counterparts. Electrophysiological studies further demonstrate that LGI1-deficient hippocampal neurons display fewer and weaker synapses. Increased RhoA activity is noted in cortical cultures from LGI1-deficient mice, and experimental evidence confirms LGI1's direct antagonism of NgR1-tumor necrosis factor receptor orphan Y (TROY) signaling.
Overall, the data suggests that LGI1 promotes synapse formation in cortical and hippocampal neurons by mitigating NgR1 signaling pathways.
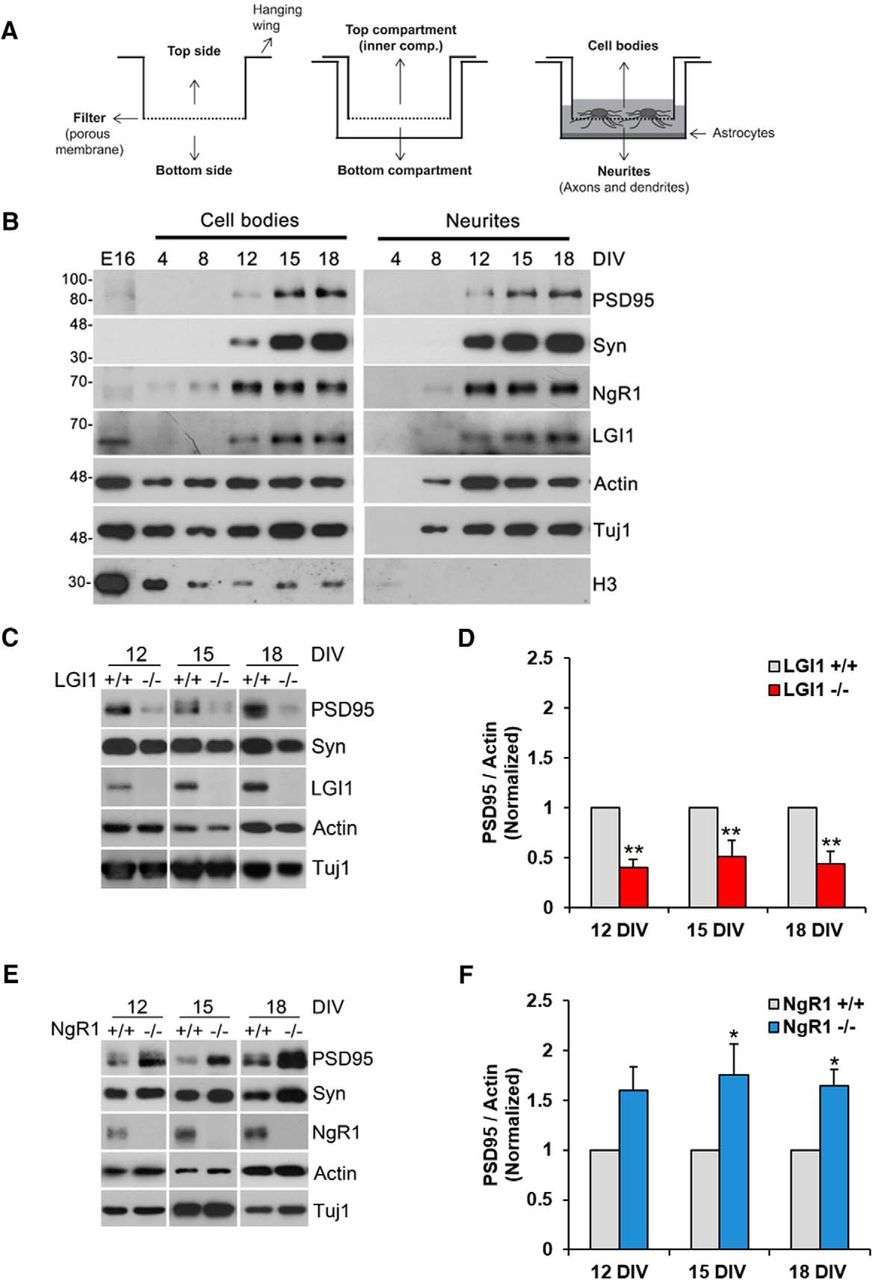 Fig.2 NgR1 and LGI1 regulate synaptic proteins in cortical neurons in vitro.
Fig.2 NgR1 and LGI1 regulate synaptic proteins in cortical neurons in vitro.Related References
- Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11(12):799-811.
- Smedfors G, Olson L, Karlsson TE. A Nogo-like signaling perspective from birth to adulthood and in old age: brain expression patterns of ligands, receptors, and modulators. Front Mol Neurosci. 2018;11:42.
- Kalafatakis I, Papagianni F, Theodorakis K, Karagogeos D. Nogo-A and LINGO-1: Two important targets for remyelination and regeneration. Int J Mol Sci. 2023;24(5):4479.
- Pradhan LK, Das SK. The regulatory role of reticulons in neurodegeneration: insights underpinning therapeutic potential for neurodegenerative diseases. Cell Mol Neurobiol. 2021;41(6):1157-1174.

