Thymus-Independent B Cell Activation
Related Symbol Search List
Immunology Background
Background
Thymus-independent (TI) B cell activation refers to the activation of B cells in response to antigens that do not require T cell help or interaction with the thymus. Unlike thymus-dependent (TD) B cell activation, which involves antigen presentation to T cells, TI B cell activation can occur in a T cell-independent manner. Here's an overview of the mechanisms and signaling pathways involved in TI B cell activation:
Mechanisms of Thymus-Independent B Cell Activation
| TI-1 Antigens | TI-2 Antigens |
|---|---|
|
|
Signaling Pathways Involved in Thymus-Independent B Cell Activation
| Signaling pathways | Details |
|---|---|
| 1. BCR Signaling |
|
| 2. Toll-like Receptor (TLR) Signaling |
|
| 3. BAFF (B Cell-Activating Factor) Signaling |
|
| 4. MAPK Signaling |
|
| 5. NF-κB Signaling |
|
Thymus-independent B cell activation provides a rapid and early immune response against certain pathogens but is generally less effective in generating long-term memory and high-affinity antibodies compared to thymus-dependent B cell activation. Understanding the mechanisms and signaling pathways involved in TI B cell activation is important for elucidating immune responses and developing strategies for vaccine design and immunotherapy.
Molecules Associated with Thymus-Dependent B Cell Activation
Several types of molecules are associated with thymus-independent B cell activation, and their functions can vary. Here are some examples:
| Types | Functions |
|---|---|
| Toll-like receptors (TLRs) | TLRs are pattern recognition receptors expressed on B cells and other immune cells. TLRs recognize specific pathogen-associated molecular patterns (PAMPs) and play a crucial role in initiating innate immune responses. TLRs can activate B cells directly in response to certain TI antigens, such as bacterial lipopolysaccharides (LPS) recognized by TLR4. |
| B cell receptor (BCR) signaling components | BCR is the membrane-bound immunoglobulin (antibody) expressed on B cells. BCR signaling components, including immunoglobulin heavy and light chains, Igα, Igβ, and associated kinases, are involved in the signal transduction pathway triggered by BCR engagement with TI antigens. They mediate intracellular signaling events that result in B cell activation, proliferation, and differentiation. |
| Complement receptors | B cells express complement receptors, such as CR1 (CD35) and CR2 (CD21), which can recognize complement-coated antigens. Complement receptors participate in the uptake of complement-opsonized TI antigens and enhance B cell activation by co-stimulatory signaling. |
| BAFF (B cell activating factor) | BAFF, also known as B lymphocyte stimulator (BLyS), is a cytokine produced by various cell types, including dendritic cells and macrophages. BAFF promotes B cell survival, proliferation, and antibody production. It plays a role in TI B cell activation by providing survival signals to the activated B cells. |
| CD19 | CD19 is a co-receptor expressed on B cells that modulates BCR signaling. It amplifies the BCR-mediated signals and enhances B cell activation, proliferation, and antibody production, including in response to TI antigens. |
| TACI (Transmembrane Activator and CAML Interactor | TACI is a receptor expressed on B cells that binds to BAFF and a proliferation-inducing ligand (APRIL). TACI engagement can enhance B cell activation and antibody production in response to TI antigens. |
| CD40-independent pathways | Some TI antigens can activate B cells independently of CD40-CD40L interactions, which are typically required for thymus-dependent B cell activation. These CD40-independent pathways involve various signaling molecules and adapters, such as MyD88 and TRIF, leading to B cell activation and antibody production. |
There are also a number of molecules associated with T thymus-independent B cell activation, such as LILRB1, Cd180, CD32A, Fcgr2b, FCGR2C, LILRA1, SLAMF8, TLR9, and UBD. These molecules and pathways collectively contribute to thymus-independent B cell activation, facilitating the immune response against certain classes of antigens that do not require T cell help. By recognizing and responding to TI antigens, B cells can generate rapid and early antibody responses, providing immediate defense against pathogens.
Diseases and Conditions Associated with Thymus-Independent B cell Activation
Thymus-independent (TI) B cell activation plays a crucial role in various diseases and conditions, including bacterial infections and autoimmune disorders. Here's an overview of some diseases and conditions associated with thymus-independent B cell activation:
Bacterial Infections
Thymus-independent antigens derived from bacterial components, such as lipopolysaccharides (LPS), peptidoglycans, and bacterial DNA, can directly activate B cells in a T-cell-independent manner. This activation leads to the production of antibodies against bacterial pathogens. Examples of bacterial infections where TI B cell activation is involved include pneumococcal infections, meningococcal infections, and Haemophilus influenzae infections.
Autoimmune Disorders
In certain autoimmune disorders, thymus-independent B cell activation can contribute to the production of autoantibodies that target self-antigens. This can lead to tissue damage and inflammation. Examples of autoimmune disorders where TI B cell activation may play a role include systemic lupus erythematosus (SLE), rheumatoid arthritis, and Sjögren's syndrome.
Immunodeficiency Disorders
Some immunodeficiency disorders can affect thymus-dependent B cell activation pathways, leading to a compensatory increase in thymus-independent B cell responses. This can result in an altered immune response and increased susceptibility to infections. Examples of immunodeficiency disorders where TI B cell activation may be involved include common variable immunodeficiency (CVID) and specific antibody deficiency.
Vaccination
Thymus-independent B cell activation is also important for effective vaccine responses. Certain vaccines, particularly those targeting polysaccharide antigens, rely on TI B cell activation to induce protective antibody responses. Vaccines against pathogens like Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B utilize TI B cell activation for antibody production.
Chronic Inflammatory Diseases
In some chronic inflammatory diseases, persistent or recurring exposure to certain antigens can lead to sustained thymus-independent B cell activation. This chronic activation can contribute to the production of autoantibodies and perpetuate the inflammatory response, leading to tissue damage. Examples of chronic inflammatory diseases where TI B cell activation may be involved include chronic obstructive pulmonary disease (COPD) and certain forms of interstitial lung disease.
Thymus-independent B cell activation plays a significant role in mounting immune responses against certain pathogens, but it can also contribute to dysregulated immune responses in autoimmune disorders and chronic inflammatory diseases. Understanding the mechanisms underlying TI B cell activation in these diseases is crucial for developing targeted therapeutic interventions and improving patient outcomes.
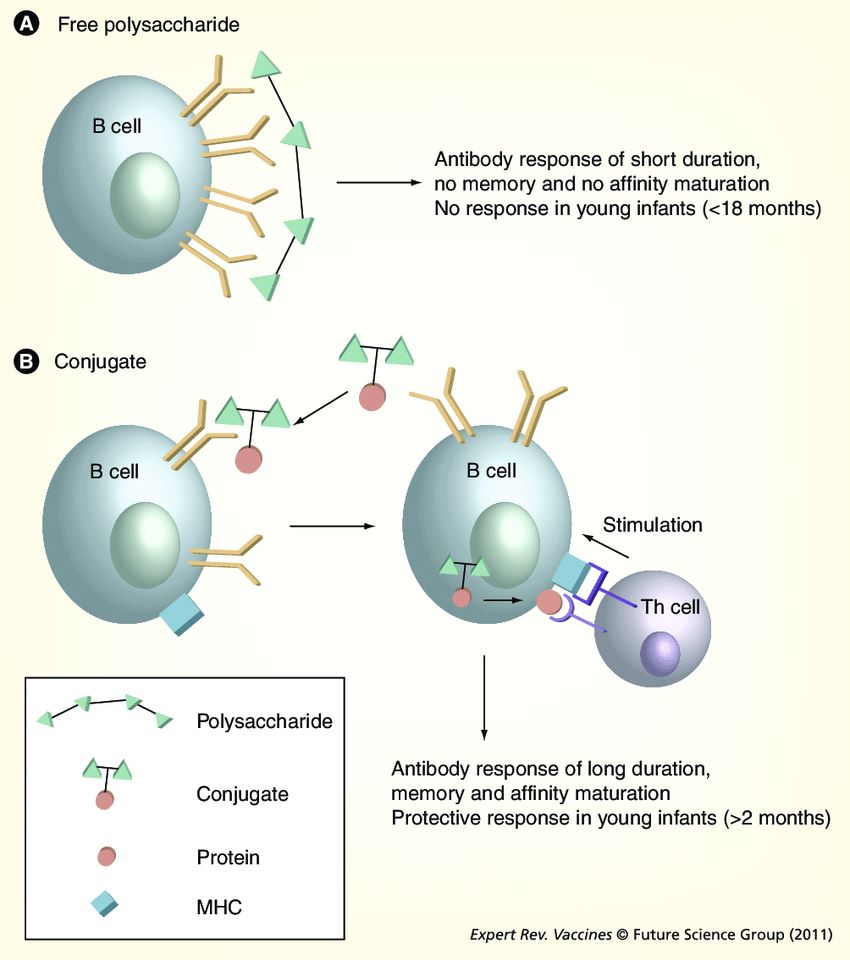 Fig.1 T-cell-independent and T-cell-dependent responses to polysaccharide and glycoconjugate vaccines. (Poolman J, et al., 2011)
Fig.1 T-cell-independent and T-cell-dependent responses to polysaccharide and glycoconjugate vaccines. (Poolman J, et al., 2011)Case Study
Case 1: Granja AG, Perdiguero P, Martín-Martín A, Díaz-Rosales P, Soleto I, Tafalla C. Rainbow trout IgM+ B cells preferentially respond to thymus-independent antigens but are activated by CD40L. Front Immunol. 2019;10:2902.
Taking into consideration the apparent contradiction of IgM+ B cells being able to respond to CD40L stimulation, but only being effectively activated by TI-1 antigens, the authors decided to perform a series of experiments to determine whether synergistic effects could be established between CD40L and the different antigens. Splenocytes were incubated with the different antigens in the presence or absence of CD40L and the levels of surface MHC II on IgM+ B cells studied through flow cytometry. A significant synergistic effect between CD40L and TNP-LPS was evident (A, B). Similarly, the incubation of splenocytes with a combination of TNP-LPS and CD40L promoted the presence of a significantly higher number of plasma cells than that observed in response to TNP-LPS or CD40L alone (C). Interestingly, these synergistic effects were never observed when CD40L was combined with TNP-KLH (A–C).
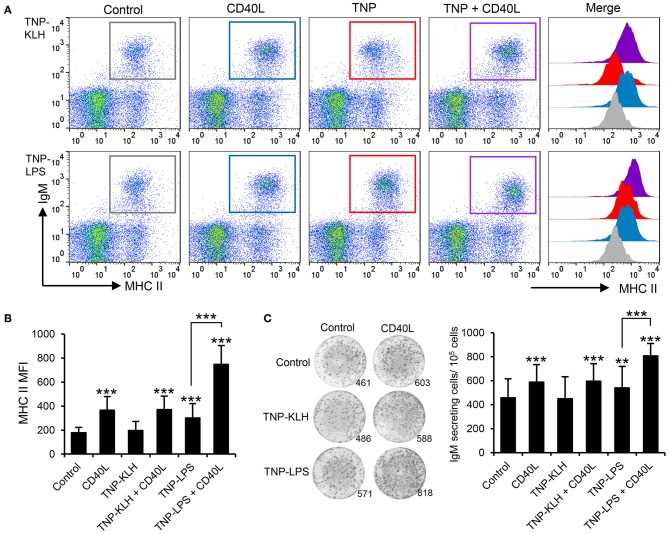 Fig.1 CD40L synergizes with TI-1 antigens to activate teleost IgM+ B cells.
Fig.1 CD40L synergizes with TI-1 antigens to activate teleost IgM+ B cells.Case 2: Fukao S, Haniuda K, Tamaki H, Kitamura D. Protein kinase Cδ is essential for the IgG response against T-cell-independent type 2 antigens and commensal bacteria. Elife. 2021;10:e72116.
Besides TI-1 Ags, such as TLR ligands, the authors sought to identify costimulating molecules that induce class switching in the TI-2 response, some of which were suggested previously. Since IgG3 is the most dominant class-switched Ig isotype produced in a TI-2 response, the authors screened various cytokines for their ability to promote the production of IgG3 in the presence of NP-Ficoll, and identified IL-1α, IL-1β, and IFNα as efficient costimuli for IgG3 production (C). These cytokines, together with NP-Ficoll, induced generation of IgG3+ cells (D), as well as IgG1+ and IgG2b+ cells to a lesser extent (E). In the presence of these cytokines, NP-Ficoll was far more potent than NP-CGG for IgG3 production, IgG3+ cell generation, Sµ-Sγ3 CSR (detectable as the Iγ3-Cµ transcript from the switch circle DNA), and the induction of Aicda transcription (C–E). NP-Ficoll or each of these cytokines alone could not induce those responses (C–E). Collectively, these results indicate that BCR signaling elicited by TI-2 Ag engagement is pivotal for the TI-2 B-cell response, namely induction of proliferation and antibody production, as well as potentiation of CSR to IgG.
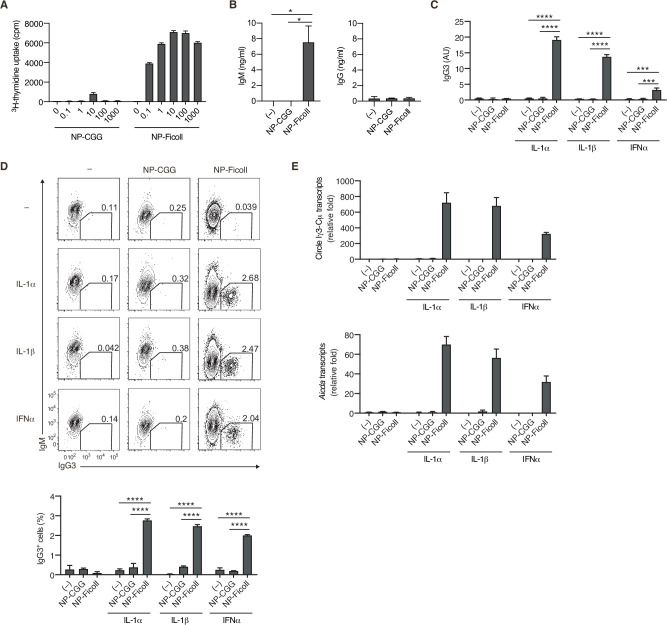 Fig.2 T-cell-independent type-2 (TI-2) antigen (Ag) distinctively induces B-cell activation and class-switch recombination (CSR) to IgG in vitro.
Fig.2 T-cell-independent type-2 (TI-2) antigen (Ag) distinctively induces B-cell activation and class-switch recombination (CSR) to IgG in vitro.References
- Granja AG, Perdiguero P, Martín-Martín A, Díaz-Rosales P, Soleto I, Tafalla C. Rainbow trout IgM+ B cells preferentially respond to thymus-independent antigens but are activated by CD40L. Front Immunol. 2019;10:2902.
- Lindroth K, Mastache EF, Roos I, Fernández AG, Fernández C. Understanding thymus-independent antigen-induced reduction of thymus-dependent immune responses. Immunology. 2004;112(3):413-419.
- Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10(3):307-322.

