IVD of Helicobacter pylori
🧪 cagA-1150H
Source: E.coli
Species: H.pylori
Tag: His&SUMO
Conjugation:
Protein Length: 918-1147 a.a.

🧪 HP0243-1191H
Source: E.coli
Species: H.pylori
Tag: His&SUMO
Conjugation:
Protein Length: 1-144 a.a.
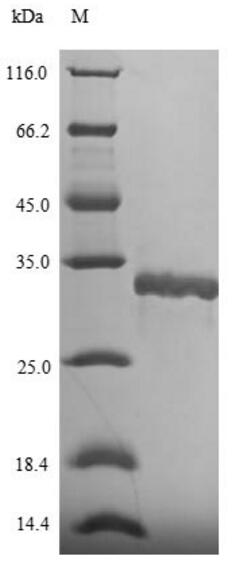
🧪 vacA-1398H
Source: E.coli
Species: H.pylori
Tag: His&SUMO
Conjugation:
Protein Length: 1038-1301 a.a.
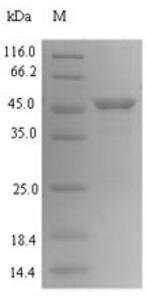
🧪 HP0887-1399H
Source: E.coli
Species: H.pylori
Tag: His
Conjugation:
Protein Length: 37-245 a.a.

🧪 GLTX1-865H
Source: E.coli
Species: Helicobacter Pylori
Tag: His&SUMO
Conjugation:
Protein Length: 1-463 aa
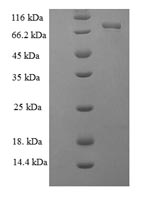
🧪 PYRF-1268H
Source: E.coli
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 1-227 aa
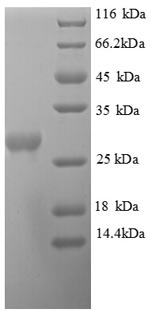
🧪 UREB-1269H
Source: E.coli
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 1-569 aa

🧪 VACA-1270H
Source: E.coli
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 37-245 aa
🧪 GLTX1-1565H
Source: Yeast
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 1-463 aa
🧪 UREA-1579H
Source: Yeast
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 1-238 aa
🧪 CAGA-1604H
Source: Yeast
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 877-1182 aa
🧪 CAGA-1882H
Source: E.coli
Species: Helicobacter Pylori
Tag: His&SUMO
Conjugation:
Protein Length: 918-1147 aa
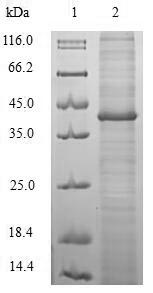
🧪 DPS-2127H
Source: E.coli
Species: Helicobacter Pylori
Tag: His&SUMO
Conjugation:
Protein Length: 1-144 aa
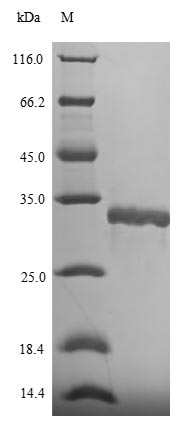
🧪 FTNA-2525H
Source: Yeast
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 1-167 aa
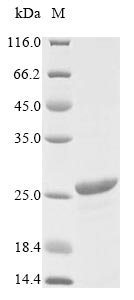
🧪 FTNA-2570H
Source: E.coli
Species: Helicobacter Pylori
Tag: His
Conjugation:
Protein Length: 1-167 aa

Helicobacter pylori (H. pylori)
Helicobacter pylori (H. pylori) is a Gram-negative bacterium that colonizes the human stomach and is associated with various gastrointestinal diseases, including peptic ulcers, gastritis, and gastric cancer. H. pylori is the only microanaerobic bacterium that can survive in the strong acid environment of the stomach and is mainly transmitted through the oral-oral and fecal-oral routes. After infection, it can cause gastrointestinal symptoms and gastritis. In severe cases, it may even develop into gastric cancer. Currently, the detection and eradication of H. pylori is a hot topic in the prevention and control of digestive system tumors. Therefore, how to accurately diagnose H. pylori infection is a prerequisite for effective prevention and control of gastric cancer. Commonly used in vitro diagnostic (IVD) methods for H. pylori infection are divided into invasive and non-invasive.
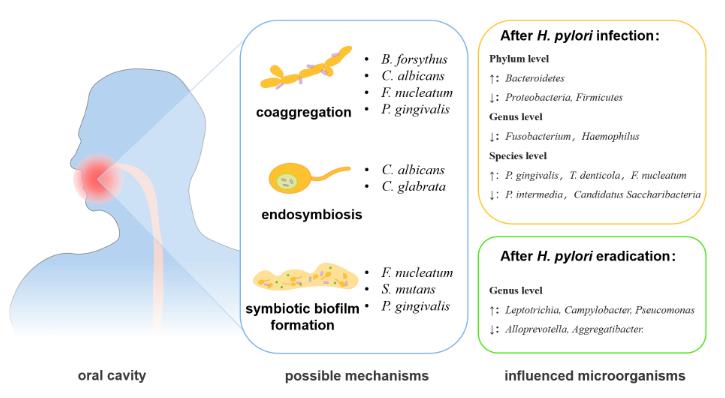 Figure 1. The changes and interactional mechanisms of H. pylori and oral microbiota. (Chen X, et al., 2022)
Figure 1. The changes and interactional mechanisms of H. pylori and oral microbiota. (Chen X, et al., 2022)Main Methods of IVD for Helicobacter pylori
- Non-invasive Inspection
- Urea breath test (UBT). The main principle is that HP can produce relatively specific urease, which can decompose urea to produce NH3 and CO2.
- Stool antigen test (SAT)
- Serological testing. These include the protein chip method, western blot (WB), rapid colloidal gold method, enzyme-linked immunosorbent assay (ELISA), and latex-enhanced immunoturbidimetric method, etc.
- Molecular Biology Technology
- Polymerase chain reaction (PCR)
- Quantitative real-time PCR (qPCR)
- LAMP
- Next-generation sequencing technology (NGS)
- Invasive Examination
- Endoscopy
- Rapid urease test (RUT)
- Bacterial culture
- Histological sections and histochemical staining microscopy
Highlights of Our Products
- Completed biological functions and efficient activity.
- It is widely used and suitable for downstream immunological experiments.
- Easy to store and transport, conducive to large-scale production and use of vaccines.
- High sensitivity, high specificity, and high purity.
- Outstanding success rate and fast development speed.
Our Outstanding Advantages
- IVD proteins can be used to test for a variety of diseases and conditions, making them valuable tools for diagnosing and monitoring health.
- Guarantee high performance, high reliability, and high consistency of protein quality, leading the industry.
- A complete IVD protein platform can provide customized services to meet different scientific research needs.
- High-quality service, high-level experiments, and reliable analysis.
In addition, Creative BioMart also offers a series of viral proteins and protein-related services to provide customers with high-quality, low-cost active recombinant proteins to meet different needs and assist in preclinical drug development.
Applications
1. Diagnostics:
Serological Tests: H. pylori proteins such as Urease, CagA, and VacA are used as antigens in serological tests to detect specific antibodies in blood samples, aiding in diagnosing H. pylori infections.
Urea Breath Test: Urease, an enzyme produced by H. pylori, is the basis for the urea breath test. In this test, patients ingest urea labeled with a carbon isotope (13C or 14C). If H. pylori is present, urease breaks down the urea, releasing labeled carbon dioxide that can be detected in the patient's breath.
2. Vaccines:
Vaccine Development: Proteins like CagA (cytotoxin-associated gene A) and VacA (vacuolating cytotoxin A) are under investigation as potential components of vaccines to prevent H. pylori infection. The goal is to elicit an immune response that can protect against colonization and associated diseases.
3. Therapeutics:
Targeted Therapy: Understanding the structure and function of H. pylori proteins can help in the design of targeted therapies. For example, inhibitors of Urease are being explored to reduce the survival of H. pylori in the acidic environment of the stomach.
Immunotherapy: Certain H. pylori proteins are being studied for their potential to modulate the immune system, which might be useful in the treatment of H. pylori-related diseases or other conditions involving immune dysregulation.
4. Research Tools:
Pathogenesis Studies: Proteins such as CagA and VacA are crucial in understanding the pathogenesis of H. pylori. CagA is delivered into host cells via a type IV secretion system, where it can interfere with multiple cellular pathways. VacA induces vacuole formation in host cells. Studying these proteins helps to elucidate how H. pylori causes disease.
Model Systems: H. pylori proteins are used in various model systems (e.g., in vitro cell cultures, animal models) to study bacterial colonization, host-pathogen interactions, and the development of gastric diseases.
5. Biotechnology:
Bioengineering: Certain properties of H. pylori proteins, like the Urease enzyme's ability to neutralize stomach acid, can be harnessed in bioengineering applications, such as designing probiotic strains or therapeutic bacteria for gastrointestinal health.
6. Drug Development:
Molecular Targets: H. pylori proteins serve as molecular targets for drug development. Inhibitors developed against these proteins can potentially treat H. pylori infections by disrupting critical bacterial functions.
7. Understanding Resistance:
Antibiotic Resistance: Studying the proteins involved in H. pylori's resistance mechanisms can inform the development of new antibiotics or treatment strategies to combat antibiotic-resistant strains.
Case Study
Case 1: Nguyen TC, Robert A, Pham THA, Vo KH, Le LD, Ma HT, Le MHT, Che TH, Nguyen HT, Truong DQ, Bontems P, Nguyen PNV. Helicobacter pylori Eradication Rate Using Stool Antigen Test in Vietnamese Children: A Prospective Multicenter Study. JPGN Rep. 2023 Oct 9;4(4):e374. doi: 10.1097/PG9.0000000000000374. PMID: 38034459; PMCID: PMC10684207.
This study assessed the diagnostic value of a monoclonal immunoassay stool antigen test (HpSA) for Helicobacter pylori (H. pylori) infection and the eradication outcomes. The HpSA is reliable for identifying H. pylori infection in epidemiological studies and assessing eradication outcomes. The low eradication rate highlights the need for an appropriate intervention strategy in Vietnamese children.
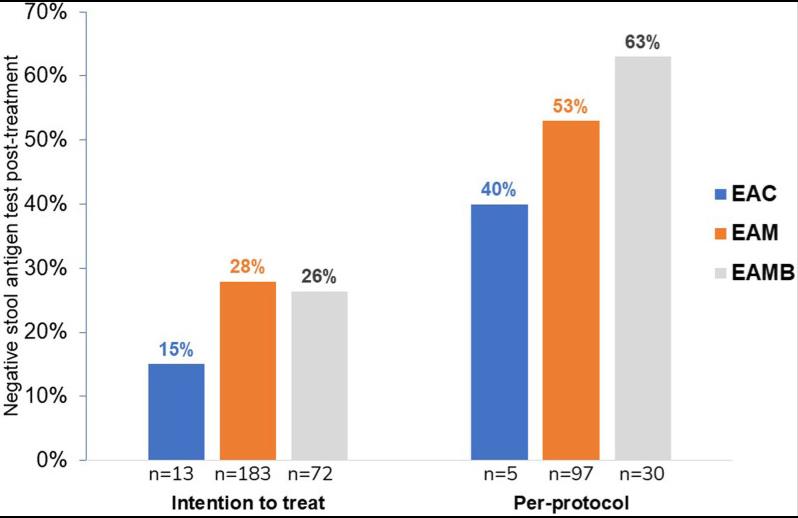 Fig2. Helicobacter pylori (H. pylori) eradication rate with EAC, EAM, EAMB, by intention to treat, and per-protocol analysis. A, amoxicillin; B, bismuth subcitrate; C, clarithromycin; E, esomeprazole; M, metronidazole.
Fig2. Helicobacter pylori (H. pylori) eradication rate with EAC, EAM, EAMB, by intention to treat, and per-protocol analysis. A, amoxicillin; B, bismuth subcitrate; C, clarithromycin; E, esomeprazole; M, metronidazole.Case 2: Krzysiek-Maczka G, Brzozowski T, Ptak-Belowska A. Helicobacter pylori-activated fibroblasts as a silent partner in gastric cancer development. Cancer Metastasis Rev. 2023 Dec;42(4):1219-1256. doi: 10.1007/s10555-023-10122-1. Epub 2023 Jul 17. PMID: 37460910; PMCID: PMC10713772.
The discovery of Helicobacter pylori (Hp) infection of gastric mucosa leading to active chronic gastritis, gastroduodenal ulcers, and MALT lymphoma laid the groundwork for understanding of the general relationship between chronic infection, inflammation, and cancer. Nevertheless, this sequence of events is still far from full understanding with new players and mediators being constantly identified. The current review concentrates on the consequences of Hp-induced increase in gastric fibroblast and myofibroblast number, and their activation towards CAFs with the emphasis to the altered communication between mesenchymal and epithelial cell compartment, which may lead to inflammation, epithelial stem cell overproliferation, disturbed differentiation, and gradual gastric cancer development.
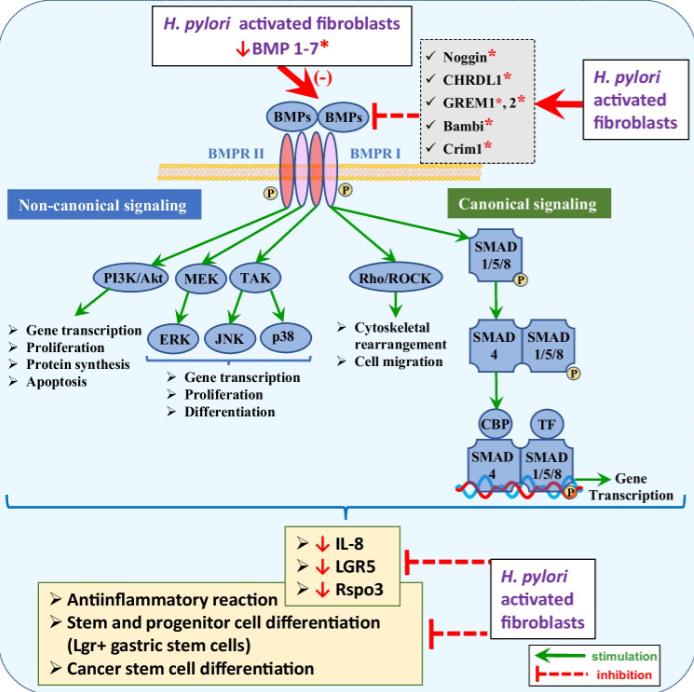 Fig. 3 The influence of Hp-infected fibroblasts on downregulation of canonical and non-canonical BMP signaling. BMPs are regulatory peptides which through the canonical, SMAD-dependent, and non-canonical, SMAD-independent signaling induce mesenchymal, gastric epithelial, and cancer stem cell differentiation. BMPs have also been reported to evoke anti-inflammatory response by inhibition of IL-8 gene expression. Hp infection leads to decreased expression of BMP1 to 7 and increased expression of BMP inhibitors such as CRIM1, CHRDL1, GREM1, and BAMBI, thus diminishing differentiation of stem and progenitor cells leading to hyperproliferation and hyperplasia accompanied with inflammatory reaction. Red asterisk denotes the factors released by activated fibroblasts/CAFs during Hp infection
Fig. 3 The influence of Hp-infected fibroblasts on downregulation of canonical and non-canonical BMP signaling. BMPs are regulatory peptides which through the canonical, SMAD-dependent, and non-canonical, SMAD-independent signaling induce mesenchymal, gastric epithelial, and cancer stem cell differentiation. BMPs have also been reported to evoke anti-inflammatory response by inhibition of IL-8 gene expression. Hp infection leads to decreased expression of BMP1 to 7 and increased expression of BMP inhibitors such as CRIM1, CHRDL1, GREM1, and BAMBI, thus diminishing differentiation of stem and progenitor cells leading to hyperproliferation and hyperplasia accompanied with inflammatory reaction. Red asterisk denotes the factors released by activated fibroblasts/CAFs during Hp infectionCase 3: Mu T, Lu ZM, Wang WW, Feng H, Jin Y, Ding Q, Wang LF. Helicobacter pylori intragastric colonization and migration: Endoscopic manifestations and potential mechanisms. World J Gastroenterol. 2023 Aug 14;29(30):4616-4627. doi: 10.3748/wjg.v29.i30.4616. PMID: 37662858; PMCID: PMC10472897.
The endoscopic manifestations and pathological features of H. pylori infection are diverse and vary with the duration of infection. In this review, the authors describe the endoscopic manifestations of each stage of H. pylori gastritis and then reveal the potential mechanisms of bacterial intragastric colonization and migration from the perspective of endoscopists to provide direction for future research on the effective therapy and management of H. pylori infection.
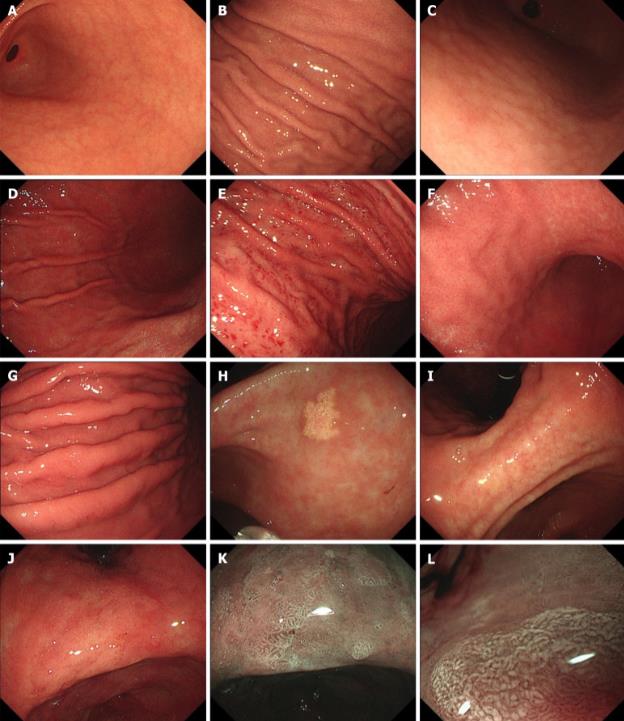 Fig4. Endoscopic features without and with Helicobacter pylori infection. A: Antrum without Helicobacter pylori infection; B: Corpus without Helicobacter pylori infection; C: Nodularity; D: Diffuse redness; E: Spotty redness; F: Mucosal swelling; G: Enlarged folds; H: Xanthoma; I: Atrophy; J: Intestinal metaplasia; K: Light-blue crest; L: White opaque substance.
Fig4. Endoscopic features without and with Helicobacter pylori infection. A: Antrum without Helicobacter pylori infection; B: Corpus without Helicobacter pylori infection; C: Nodularity; D: Diffuse redness; E: Spotty redness; F: Mucosal swelling; G: Enlarged folds; H: Xanthoma; I: Atrophy; J: Intestinal metaplasia; K: Light-blue crest; L: White opaque substance.Reference
- Chen X, Wang N, Wang J, et al. (2022). The interactions between oral-gut axis microbiota and Helicobacter pylori[J]. Frontiers in Cellular and Infection Microbiology. 12: 914418.

