Recombinant Protein and Its Expression Systems
What is recombinant protein? Proteins are one of the most important biological molecules for life. And these proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli and transporting molecules from one location to another. However, when we want to analyze the structure, function, mechanism, pathway or other facts of these proteins, we will find that the quantities are not sufficient from nature source. In that case we need to produce high quantity and purity proteins in a short time and low cost in our lab. Thus, recombinant protein technology is required. Recombinant protein is a manipulated form of protein, which is generated in various ways to produce large quantities of proteins, modify gene sequences and manufacture useful commercial products. Recombinant protein is encoded by recombinant DNA, which has been cloned in a system that supports expression of the gene and translation of mRNA. The recombinant DNA, usually the cDNA sequence of the target protein, is designed to be under the control of a well characterized promoter and express the target protein within the chosen host cell to achieve high-level protein expression. Modification of the gene by recombinant DNA technology can lead to expression of a mutant protein or a large quantity of protein.
Choosing an appropriate protein expression system is the key to the success of recombinant protein expression. Several factors need to be taken into consideration, including target protein property, intended application, protein yield and cost. Furthermore, challenges exist for many protein expression projects, especially for large protein, membrane protein, nuclear protein and proteins with heavy post-translational modifications.
Nowadays, there are several expression systems for use. Different systems have different features and applications. Here, we will introduce four systems which are commonly used in research and industrial. They are bacteria expression system, yeast expression system, baculovirus expression system and mammalian expression system.
 Fig1. Four Expression Systems
Fig1. Four Expression Systems
- Bacteria Expression System
E.coli expression system is the primary use and most mature expression system. The main method is transfer a vector which inserted target DNA fragment to host cell. And then induce protein expression by IPTG.
As the earliest development and the most widely used classical expression system, the E. coli expression system has the advantages of clear genetic background, fast breeding, low cost, high expression, easy purification of the product, good stability, strong anti-pollution ability and wide application range. However, there are many shortcomings in the prokaryotic expression system: not all proteins are soluble. Incorrectly folded proteins formed in cytoplasm can form insoluble aggregates called inclusion bodies, which leads to the difficult purification. Moreover, the post-translational modification processing of the prokaryotic expression system is imperfect and the biological activity of the expressed product is low.
Thus, other more sophisticated systems are also being developed; such systems may allow for the expression of proteins previously thought impossible in E.coli, for example, glycosylated proteins.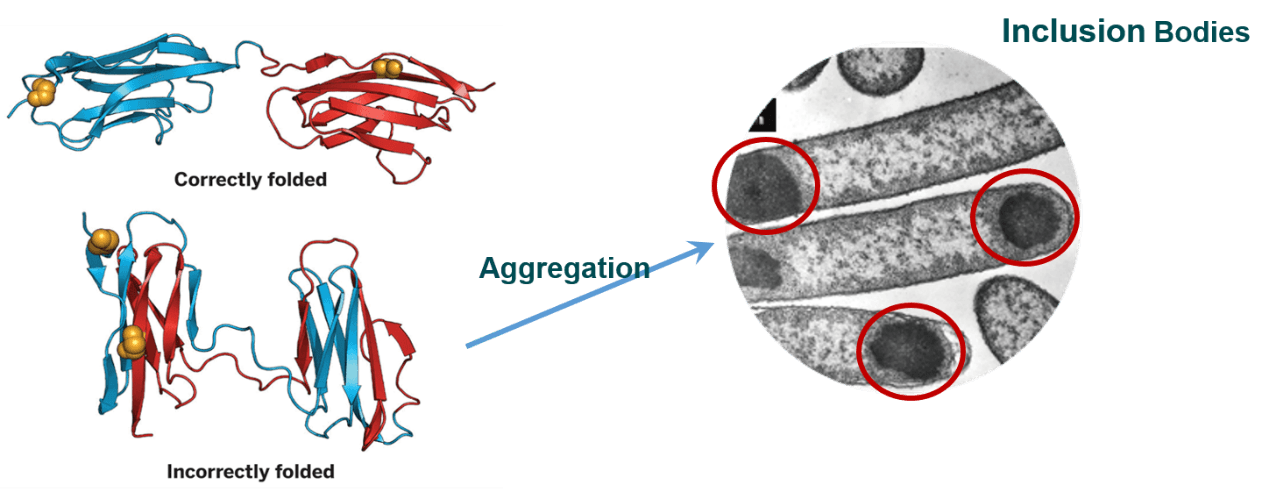 Fig2. Incorrectly folded and inclusion bodies
Fig2. Incorrectly folded and inclusion bodies - Yeast Expression System
Yeast expression system as a new exogenous protein expression system, contained merits from both prokaryotic and eukaryotic expression system. It is being widely used in the field of genetic engineering. Complete gene sequence of Saccharomyces cerevisiae are sequenced in 1996. The use of S.cerevisiae in the brewing and bread industry has been known for thousands of years and is considered to be GRAS (generally recognized as safe) creatures that do not produce toxins and has also been recognized by FDA. Thus, the productions expressed from yeast system need not a lot of host safety experiments.
However, compared with the new yeast system, S.cerevisiae expression system is not suitable for high-density culture; lacking strong and strict regulation promoter. And the secretion efficiency is low. Especially, many target protein which molecular weight greater than 30kD almost does not secrete.
Later, people have developed fission yeast and methanol yeast expression system. Among them, the methanol yeast expression system is the most widely used yeast expression system. Nowadays, mainly used methanol yeasts are H Polymorpha, Candida Bodini and Pichia Pastris. Pichia Pastoris is the most popularity tool. Most of the methanol yeast contains the methanolic yeast oxidase gene-1 (AOX1). The exogenous gene was expressed under the action of the promoter (PAOX1). PAOX1 is a strong promoter using glucose or glycerol as carbon source. The expression of AOX1 gene in methanol yeast was usually inhibited. And PAOX1 could be activated when methanol was the sole carbon source. Therefore, the expression of AOX1 gene could be increased under the control of the gene. The use of methanol yeast to express exogenous protein production is often up to grams. Compared with S.cerevisiae, its translation is closer to mammalian cells and does not undergo hyperglycosylation.
- Baculovirus Expression System
The insect expression system is a widely used eukaryotic expression system that has the ability to translate and modify foreign proteins similar to higher eukaryotes. The expression of exogenous protein in the insect cell system by recombinant baculovirus is a more popular expression method. The protein level is up to 1 ~ 500mg / L. But it is restricted and influenced by many factors such as culture medium, oxygen supply and logarithmic growth and so on.
Baculovirus is the largest group of known insect viruses and is the earliest, most studied and applicable insect virus. The baculovirus genome is a single closed circular double-stranded DNA molecule with a size of 80-160 kb. Its genome can be replicated and transcribed in insect nuclei. DNA replication is assembled in the bark of the baculovirus, which has a great flexibility and could accommodate large fragments of foreign DNA insert. It is the ideal carrier for the expression of large fragments of DNA.
The main advantages of baculovirus system include:
- Recombinant protein has complete biological function, such as protein correct folding and disulfide bond;
- Post-translation modification;
- High level of expression, up to 50% of the total protein amount;
- Accommodate large insert protein;
- Simultaneously express multiple genes.
The main drawback is that exogenous protein expression is under the control of the very late viral promoter, where the cells begin to die due to viral infection.
Insect expression system is normally used for production of membrane proteins, although the glycosylations may be different from those found in vertebrates. In general, it is safer to use than mammalian virus, since it has limited host range and does not infect vertebrates without modifications. - Mammalian Expression System
Recombinant proteins expressed in mammalian cell commonly use plasmid transfection and viral vector infection. Stable cell line using plasmid transfection takes several weeks or even months, while the virus vector can quickly infect cells within a few days.
Depending on the temporal and spatial differences in protein expression, the expression system can be divided into transient, stable and induced expression systems. Transient expression system refers that host cell cultures without selection pressure and exogenous vector gradually lost while cell division. The target protein expression duration is short. The advantage of transient expression system is simple and short experimental period. Stable expression system means that the carrier DNA replicate and express long time stably in host cell. Due to the need of select resistance and pressure steps, stable expression is relatively time-consuming and laborious. Induction expression system refers that the target gene begins to express when induced by foreign small molecules. The use of heterologous promoters, enhancers and amplifiable genetic markers can increase protein production.
The mammalian expression system has unique advantage in protein initiation signals, processing, secretion, glycosylation and is suitable for expressing intact macromolecules.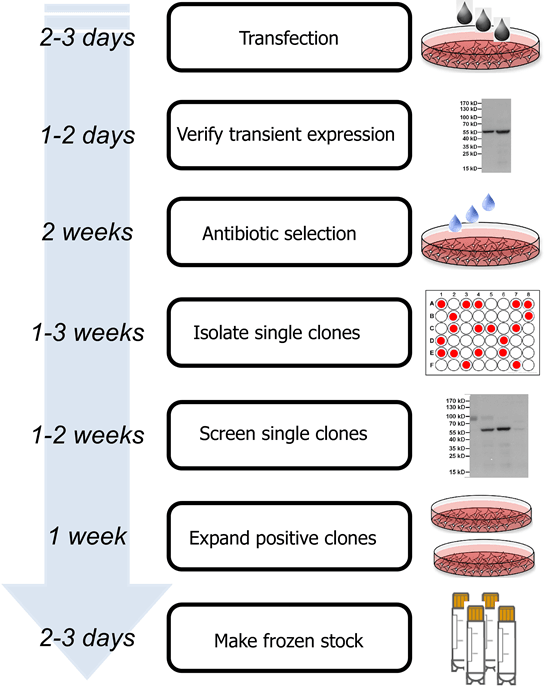 Fig3.Stable cell line development
Fig3.Stable cell line developmentThe foreign protein produced by mammalian cells, which is closer to the native protein, has much more activity than proteins produced by prokaryotic expression system or by other eukaryotic expression system, such as yeast and insect cells. The disadvantage of the technique is complicated, high requirement, low yield and sometimes viral infections exist.
Above all, there are advantages and disadvantages for all these expression systems. The advantage of E.coli and yeast expression system is high expression level and low cost. But their modification systems are different from insect cell and mammalian cells. Protein produced by mammalian cell is similar with native protein. However, the disadvantage is low expression level and complicated operation. The biological activity and immunogenicity of the recombinant proteins produced by different expression systems are sometimes different due to the diverted post-translational processing. Therefore, when considering which expression system to choice, we need take a variety of factors into consideration, such as whether the target protein has toxic, whether the protein need biological activity, whether the glycosylation is needed for the protein and cost efficiency, yield, purification, safety and so on. Supported by experienced experimental knowledge and consider all these factors, our expertise will make suitable determination and choice.
Table1. Simply Compare among Expression Systems
| Bacterial Expression System | Yeast Expression System | Insect Expression System | Mammalian Expression System | |
| Speed | ★★★★ | ★★★ | ★★ | ★ |
| Yield | ★★★ | ★★★★ | ★★ | ★ |
| PTM (relative to human) |
★ | ★★ | ★★★ | ★★★★ |
| Cost | ★★★★ | ★★★ | ★★ | ★ |
| Application | Prokaryotic protein, simple eukaryotic protein | Intracellular/Secreted Protein, Disulfide-bonded protein, Glycosylated protein | Membrane protein, Large-size protein, Viral vaccines, signaling proteins, cytokines, kinases |
Complex eukaryotic protein, Protein need accurate PTM |
We provide all protein expression system services in our company. If you have any questions on recombinant protein and expression system choice, welcome to contact us for details!

