Affinity Chromatography
🧪 COP08
Source:
Species:
Tag: Non
Conjugation:
Protein Length:

🧪 COP28
Source:
Species:
Tag: Non
Conjugation:
Protein Length:

🧪 Thio-001A
Source:
Species:
Tag: Non
Conjugation:
Protein Length:

🧪 Phosphate-001C
Source:
Species:
Tag: Non
Conjugation:
Protein Length:
$1,038.40
$1,298
/ 25ml$1,598.40
$1,998
/ 50ml
🧪 Ni-NTA-001A
Source:
Species:
Tag: Non
Conjugation:
Protein Length:
🧪 Heparin-27A
Source:
Species:
Tag: Non
Conjugation:
Protein Length:
🧪 NHS-001A
Source:
Species:
Tag: Non
Conjugation:
Protein Length:
Background
Overview
Affinity Chromatography is a biochemical separation technique widely used in the separation and purification of proteins, nucleic acids, cells, and other biological macromolecules.
The basic principle of affinity chromatography is to immobilize ligands with specific affinity onto solid carriers to form affinity adsorbents. When the mixed sample is passed through a chromatogram column containing this adsorbent, the target molecule will bind specifically to its ligand, while other non-specifically bound molecules will be eluted. Subsequently, by changing the composition or conditions of the eluent (such as pH, ionic strength, the addition of competitive ligands, etc.), the target molecules can be dissociated from the adsorbent to achieve purification.
Ligand Types
Biospecific ligand. Biospecific ligands are naturally occurring molecules that have a high degree of affinity and specificity with their target molecules. These ligands are usually derived from living organisms, such as proteins, polysaccharides, etc.
Pseudospecific ligand. Pseudospecific ligands are not based on natural interactions between biomolecules, but are artificially designed and synthesized. These ligands can simulate interactions between biomolecules for purification of specific target molecules.
Synthetic ligands. Synthetic ligands are prepared by chemical synthesis methods, and they can be small molecule compounds or polymers. The design and selection of synthetic ligands can be optimized according to the characteristics of the target molecule to improve the selectivity and efficiency of purification.
Affinity tag. An affinity tag is a special ligand that is introduced to the N-terminal or C-terminal of a target protein through genetic engineering techniques. These tags can be short peptide sequences or specific protein domains that bind to specific affinity ligands to enable purification of the target protein.
Matrix Types
Solid carriers in affinity chromatography, also known as substrates or supports, are materials used to immobilize affinity ligands, which provide a stable platform for the affinity ligands to capture target molecules during the chromatography process. The choice of solid carrier has an important influence on the efficiency and effect of affinity chromatography. Here are some common solid carrier types:
Agarose matrix. Agarose is a natural polysaccharide widely used in affinity chromatography. It has good biocompatibility, chemical stability and mechanical strength. Agarose matrices usually have a macroporous structure, which facilitates rapid molecular diffusion and high binding loads.
Polyacrylamide matrix. Polyacrylamide is a synthetic polymer that is often used to make affinity chromatography media. It has high mechanical strength and chemical stability and is suitable for the purification of a variety of biomolecules.
Silicone matrix. Silica gel is an inorganic material with good chemical and thermal stability. It is commonly used for affinity chromatography of small molecules, but may be less suitable for macromolecular biomolecules, such as proteins, due to their small pore sizes.
Magnetic bead matrix. Magnetic beads are a new type of affinity chromatography carrier made of magnetic materials such as iron oxides. Magnetic beads can be quickly separated by magnetic fields and are easy to operate, suitable for high-throughput and automated purification processes.
Cellulose matrix. Cellulose is a kind of natural polymer with wide sources and low cost. It can be chemically modified to introduce different functional groups for fixing affinity ligands.

Key Steps
Ligand selection and immobilization. The selection of the right ligand is the key to the success of affinity chromatography. The ligand must have a highly specific affinity to the target molecule and be able to be immobilized without affecting the biological activity of the target molecule.
Sample preparation and loading. Sample handling is required to minimize nonspecific adsorption while protecting the activity of the target molecule. Usually, the sample needs to go through the appropriate dilution, buffer exchange and other pretreatment steps.
Elution and collection. Elution is one of the most critical steps in affinity chromatography. The suitable elution conditions can effectively dissociate the target molecules from the affinity adsorbent while minimizing the elution of non-target molecules.
Regeneration and storage of chromatographic columns. After the completion of one chromatographic process, the chromatographic column needs to be properly regenerated for the next use. At the same time, the correct storage of chromatographic column is also the key to ensure its performance.
Applications
- Biopharmaceuticals. Affinity chromatography is a key technique in the biopharmaceutical industry for the isolation and purification of biologic drugs, especially for the purification of monoclonal antibodies (mAbs), recombinant proteins, vaccines, enzymes, and other biological products. For example, Protein A and Protein G affinity chromatography is often used to purify antibodies from cell cultures.
- Proteomics and protein research. Affinity chromatography is used for purification, identification and functional study of proteins. With specific affinity ligands, target proteins can be specifically captured for subsequent structural and functional analysis.
- Gene therapy. In the field of gene therapy, affinity chromatography is used to purify viral vectors such as adeno-associated viruses (AAV) and adenoviruses, which are used for in vivo and in vitro gene therapy and for the development of tumor vaccines.
- Development of diagnostic reagents. Affinity chromatography also plays an important role in the development of diagnostic kits, such as for the purification of antibodies or other binding molecules used to detect specific biomarkers.
- Biotechnology. In the field of biotechnology, affinity chromatography is used for the production of biocatalysts, such as industrial enzymes, as well as for the production of biopesticides and biofertilizers in agricultural biotechnology.

Case Study
Case Study 1: m7GTP Agarose (m7GTP-001A)
The Fragile X-related protein-1 (FXR1) gene is highly amplified in ovarian cancer patients, and this amplification is associated with increased expression of FXR1 mRNA and protein. The expression of FXR1 is directly related to the survival and proliferation of cancer cells. The Surface Translation Sensing (SUnSET) experiment showed that FXR1 enhanced the overall translation of cancer cells. The reversed phase protein Array (RPPA) revealed that cMYC is a key target for FXR1. Mechanically, FXR1 binds to the au rich element (ARE) in the 3' untranslated region (3' utr) of cMYC and stabilizes its expression. In addition, the RGG domain in FXR1 interacts with the eIF4A1 and eIF4E proteins. These two interactions of FXR1 lead to cyclization of cMYC mRNA and promote recruitment of eukaryotic translation initiation factors to translation initiation sites.
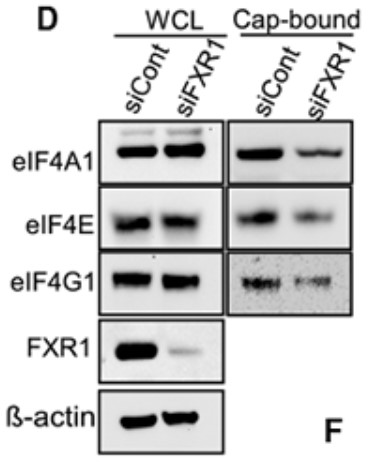
(Jasmine George, 2021)
Fig1. HeyA8 cells were transfected with control siRNA or FXR1 siRNAs for 48 h, and lysates were incubated with m7GTP (5′mRNA cap analog).
Case Study 2: Protein G Agarose High Flow Resin (COP28)
Age-related changes in human T-cell populations are important factors in immune aging. In particular, terminally differentiated CD8+ effector memory CD45RA+ TEMRA cells and their subpopulations are characteristic of cellular senescence, accumulating in the elderly and increasing in age-related chronic inflammatory diseases. Detailed T-cell analysis of individuals over 65 years of age showed high inter-individual variation in the CD8+ TEMRA population. CD8+ TEMRA ratio was positively associated with cytomegalovirus (CMV) antibody levels, but not with chronological age. Analysis of more than 90 inflammatory proteins showed that plasma TRANCE/RANKL levels were associated with several differentiated t cell populations, including CD8+ TEMRA and its CD28- subgroup. Given the strong potential of CD8+ TEMRA cells as biomarkers of immune aging, the researchers used sulfite deep amplicon sequencing to match their frequency in flow cytometry to CpG site methylation levels, and developed a computational model to predict the proportion of CD8+ TEMRA cells in whole blood genomic DNA.
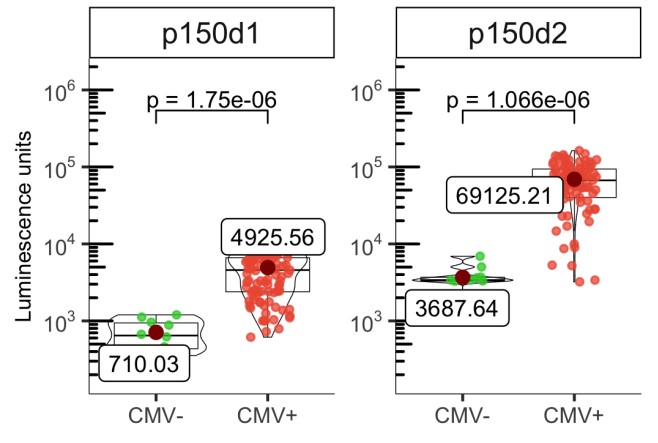
(Ahto Salumets, 2022)
Fig2. The levels of anti‐p150d1 and p50d2 antibodies in CMV positive and negative individuals are shown as luminescence units (LU) of luciferase enzyme activity as boxplots.
Case Study 3: Thiopropyl Agarose (Thio-001A)
Protein palmitoylation is a cellular process that occurs at the membrane-cytoplasmic interface and is coordinated by members of the DHHC enzyme family, which plays a key role in regulating various cellular functions. The M2 protein of influenza virus is acylated on the amphiphile helix near the membrane and can be used as a model to study the complex signals that control acylation and its interaction with the homologous enzyme DHHC20. This study shows that altering the biophysical properties of the amphipathic spiral, particularly by shortening or disrupting it, leads to a substantial reduction in M2 palmitoylation, but does not completely abolish the process. Interestingly, DHHC20 showed enhanced affinity for certain M2 mutants compared to the wild-type M2. Molecular dynamics simulations revealed the interaction between helical amino acids and DHHC20's catalytic DHHC and TTXE motifs. The results suggest that the binding of M2 to DHHC20, although not highly specific, is mediated through the necessary contact and may trigger the transfer of fatty acids.
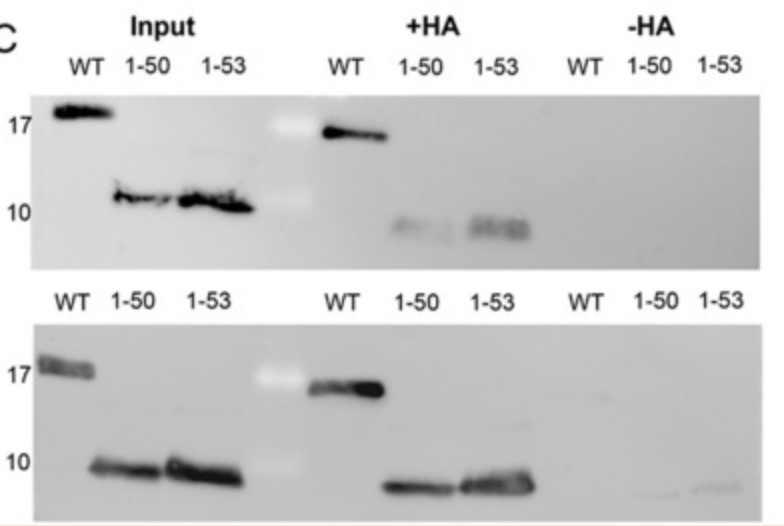
(Xiaorong Meng, 2023)
Fig3. S-acylation: M2 wt and the mutants were expressed in 293 T cells.
Advantages
- Wide ranges of products. In the choice of ligands and substrates associated with affinity chromatography, we offer a wide range of types for you to choose from. Including various ligand proteins cross-linked on AGAR or magnetic beads.
- Excellent product quality. Superb technology enables our products to assist higher purification efficiency and recovery, suitable for the purification of a variety of samples.
- Efficient and convenient. The pre-activated matrix provided eliminates many chemical activation steps and problems, making it safer and easier for customers to use.
- Customized services. We can provide customized affinity fillers and services to meet the requirements of different biological processes according to the specific needs of customers.
Creative BioMart can provide various types of affinity chromatography related products to meet customer’s different research goals. You can also let us know if you have any customized requirements. Please contact us for more product details.
References
- George J.; et al. RNA-binding protein FXR1 drives cMYC translation by recruiting eIF4F complex to the translation start site. Cell Rep. 2021;37(5):109934.
- Salumets A.; et al. Epigenetic quantification of immunosenescent CD8+ TEMRA cells in human blood. Aging Cell. 2022;21(5):e13607.
- Meng X.; et al. The role of an amphiphilic helix and transmembrane region in the efficient acylation of the M2 protein from influenza virus. Sci Rep. 2023;13(1):18928.