Nectin and Nectin-like Ligand/Receptor Co-Signaling Molecules
Related Symbol Search List
Immunology Background
Background
About Nectin and Nectin-like Ligand/Receptor Co-Signaling Molecules
Nectins and Nectin-like (Necl) molecules are cell adhesion molecules that play crucial roles in cell-cell interactions and signaling. They belong to the immunoglobulin superfamily and are involved in various physiological and pathological processes. The Nectin family consists of four members: Nectin-1, Nectin-2, Nectin-3, and Nectin-4. The Necl family comprises five members: Necl-1, Necl-2, Necl-3, Necl-4, and Necl-5. These molecules can interact with each other and with their respective receptors, collectively known as Nectin and Necl-like (Necl-Like) receptors, to mediate adhesion and co-signaling events.
Nectin and Nectin-like molecules are involved in costimulatory signaling interactions with their receptors. These costimulatory interactions can amplify or modulate immune responses and help regulate immune cell activation and effector functions. Examples of costimulatory signaling receptors include CD226, CD96, and TIGIT, which bind to specific Nectin or Nectin-like ligands.
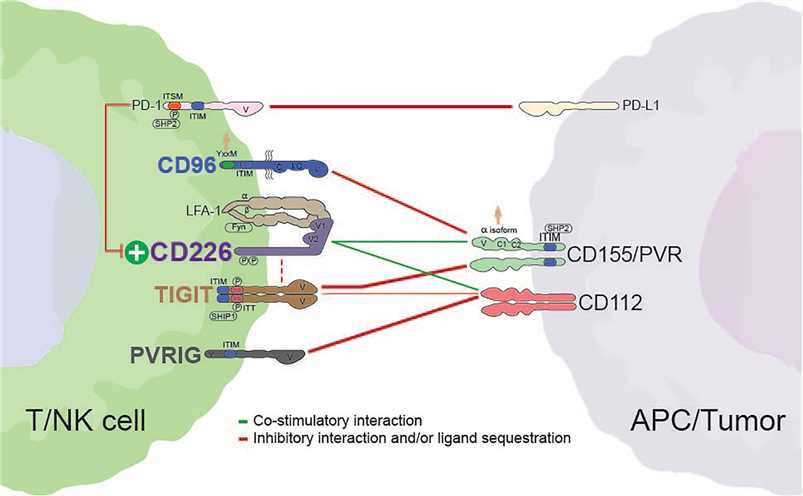 Fig.1 Interactions between members of the CD226 axis. Dashed red lines indicate the potential for cis interactions/inhibition. (Conner M, et al., 2022)
Fig.1 Interactions between members of the CD226 axis. Dashed red lines indicate the potential for cis interactions/inhibition. (Conner M, et al., 2022)The Co-signaling Molecules Associated with Nectins and Nectin-Like Ligands/Receptors
| Key molecules | Functions |
|---|---|
| CD226 (DNAM-1, DNAX accessory molecule-1) |
|
| CD96 (TACTILE) |
|
| CRTAM (Class-I-restricted T cell-associated molecule) |
|
| Pvrl2 (Nectin-2, CD112) |
|
| PVRL3 (Nectin-3, CD113) |
|
| PVR (CD155) |
|
| PVRIG (Poliovirus receptor-related immunoglobulin domain-containing protein) |
|
| TIGIT (T cell immunoreceptor with Ig and ITIM domains) |
|
Co-signaling Pathways Involving Nectin and Nectin-like Ligand/Receptor Proteins
While Nectins and Nectin-like ligand/receptor proteins primarily function as cell adhesion molecules, their interactions with co-signaling molecules can modulate immune responses through various signaling pathways. Here are some co-signaling pathways involving Nectin and Nectin-like ligand/receptor proteins:
| Pathways | Roles | |
|---|---|---|
| CD226 (DNAM-1) Co-signaling Pathway |
|
|
| TIGIT Co-signaling Pathway |
|
|
| PVRIG Co-signaling Pathway |
|
|
Overall, the co-signaling pathways involving Nectin and Nectin-like ligand/receptor proteins can have a significant impact on immune cell activation, effector functions, and immune regulation. The activation or inhibition of these pathways through interactions with CD226, TIGIT, and potentially PVRIG can fine-tune immune responses, contributing to immune homeostasis, immune tolerance, and immune surveillance.
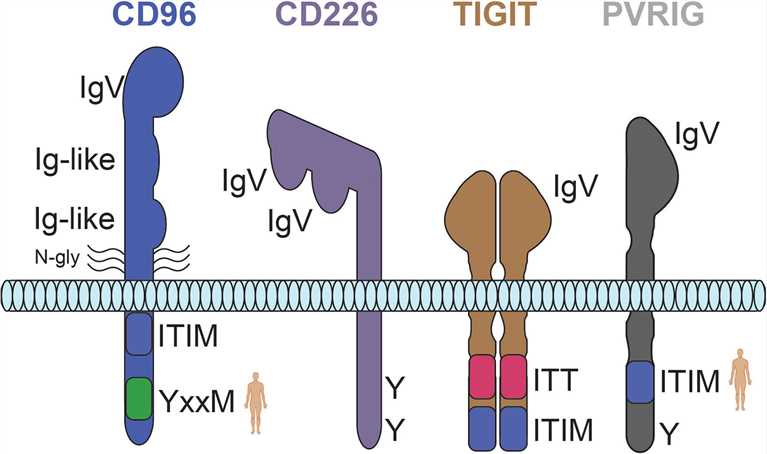 Fig.2 Predicted structures for the CD226 axis receptors CD96, CD226, TIGIT, and PVRIG. (Conner M, et al., 2022)
Fig.2 Predicted structures for the CD226 axis receptors CD96, CD226, TIGIT, and PVRIG. (Conner M, et al., 2022)The weight of each line is representative of the relative strength of interaction. Human silhouettes signify that a motif is not present in rodents. N-gly, n-linked glycosylation; Y, tyrosine residue; ITT, immunoglobulin tail tyrosine motif; ITIM, immunoreceptor tyrosine-based inhibition motif.
Role of Co-signaling Molecules Associated with Nectins and Ncl Ligands/Receptors in Different Diseases
The co-signaling molecules associated with Nectins and Nectin-like ligands/receptors have been implicated in various diseases due to their roles in immune regulation and cellular interactions. Here's an overview of their involvement in different diseases:
Cancer
- Dysregulation of co-signaling molecules, such as CD226, CD96, TIGIT, and PVR, has been observed in cancer.
- CD226 and CD96 interactions with their ligands can modulate anti-tumor immune responses, and their manipulation is being explored in cancer immunotherapy.
- TIGIT, an inhibitory receptor, is often upregulated in tumors, leading to immune suppression. Targeting TIGIT signaling is being investigated as a strategy to enhance anti-tumor immune responses.
- PVR is frequently expressed on tumor cells, and its interactions with co-signaling receptors can influence tumor immune evasion mechanisms.
- Therapies targeting these co-signaling molecules are being developed, including antibodies and chimeric antigen receptor (CAR) T cell therapies.
Autoimmune Diseases
- Altered expression or function of co-signaling molecules, such as CD226, TIGIT, and CRTAM, have been implicated in autoimmune diseases.
- In autoimmune disorders, aberrant immune activation contributes to the destruction of self-tissues.
- Modulating the signaling pathways associated with these co-signaling molecules is being explored as a potential therapeutic approach to restore immune balance and control autoimmune responses.
Infectious Diseases
- Co-signaling molecules, such as CD226 and TIGIT, play roles in immune responses against infectious agents.
- CD226 engagement can enhance immune cell functions, including cytokine production and cytotoxicity, aiding in the clearance of pathogens.
- TIGIT, on the other hand, can dampen immune responses, potentially limiting immunopathology during infections.
- The interplay between these co-signaling molecules and infectious agents influences the outcome of immune responses and can impact the course of infectious diseases.
Chronic Inflammation
- In chronic inflammatory conditions, dysregulated immune responses can lead to persistent inflammation and tissue damage.
- Co-signaling molecules, including CD226, TIGIT, and PVR, are involved in modulating immune activation and tolerance.
- Imbalances in their expression or interactions may contribute to the perpetuation of chronic inflammation.
- Targeting these co-signaling pathways holds promise for therapeutic interventions aimed at controlling chronic inflammatory disorders.
It's important to note that the roles of these co-signaling molecules in diseases are still an active area of research, and their precise contributions can vary depending on the specific disease context. Further studies are needed to fully understand their mechanisms and potential as therapeutic targets in various disease settings.
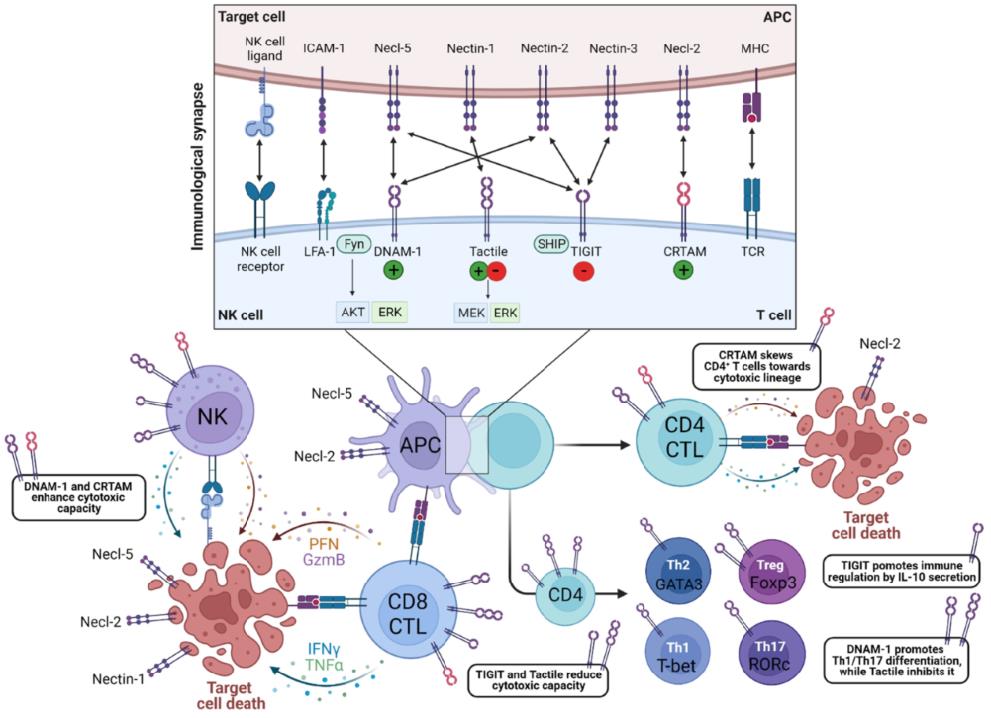 Fig.3 Overview of DNAM-1-, CRTAM-, Tactile- and TIGIT-triggered immune effector functions. (Hermans D, et al., 2023)
Fig.3 Overview of DNAM-1-, CRTAM-, Tactile- and TIGIT-triggered immune effector functions. (Hermans D, et al., 2023)Case Study
Case 1: Peng YP, Xi CH, Zhu Y, et al. Altered expression of CD226 and CD96 on natural killer cells in patients with pancreatic cancer. Oncotarget. 2016;7(41):66586-66594.
The authors evaluated the expression of CD155 in 88 pancreatic cancer tissues and 33 adjacent tissues using IHC. The results showed that the average percentage of CD155-positive cells in PC tissues and adjacent tissues was 92.64% (range, 40%-95%) and 39.52% (range, 0%-90%) respectively; the staining intensity score for CD155 was close to 3 in PC tissues and 1-3 in the adjacent tissues (A-F). The IHC scores for CD155 in PC tissues were remarkably higher than those in adjacent tissues (G). Furthermore, the level of CD155 expression did not differ significantly according to patient clinicopathological features or tumor characteristics/tumor progression, and CD155 was overexpressed in almost all the PC examples. These data indicate that CD226- and/or CD96-expressing NK cells have potential for the immune treatment of PC.
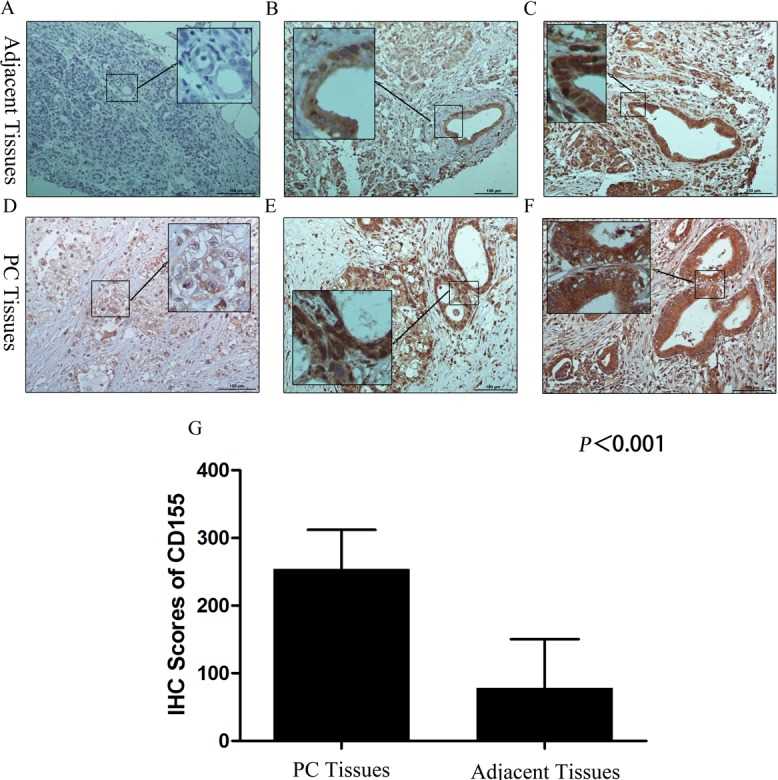 Fig.1 CD155 (PVR) expression in pancreatic cancer and adjacent tissues.
Fig.1 CD155 (PVR) expression in pancreatic cancer and adjacent tissues.Case 2: Bachelet I, Munitz A, Mankutad D, Levi-Schaffer F. Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): a novel interface in the allergic process. J Biol Chem. 2006;281(37):27190-27196.
The authors aimed at determining the susceptibility of CD226 costimulatory signaling to inhibition by immune inhibitory receptors. To test this, we have treated cells with a bispecific antibody linking CD300a to IgE, which was shown to inhibit mast cell degranulation at varying concentrations prior to FcϵRI-activation. The augmented activation resulting from CD226 engagement was significantly reduced at 1 μg/ml bispecific antibody and was completely blocked at 10 μg/ml (A). The FcϵRI/CD226-induced cytosolic calcium influx followed this exact inhibitory pattern at 1 μg/ml and 10 μg/ml (B).
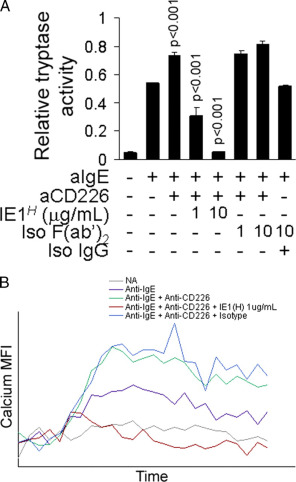 Fig.2 CD226-induced costimulation is blocked by linking IgE and CD300a.
Fig.2 CD226-induced costimulation is blocked by linking IgE and CD300a.References
- Hermans D, van Beers L, Broux B. Nectin Family Ligands Trigger Immune Effector Functions in Health and Autoimmunity. Biology. 2023; 12(3):452.
- Conner M, Hance KW, Yadavilli S, Smothers J, Waight JD. Emergence of the CD226 Axis in Cancer Immunotherapy. Front Immunol. 2022;13:914406.
- Bachelet I, Munitz A, Mankutad D, Levi-Schaffer F. Mast Cell Costimulation by CD226/CD112 (DNAM-1/Nectin-2): A Novel Interface in the Allergic Process. J Biol Chem. 2006;281(37):27190-27196.

