What is IRAK2 Protein
The IRAK2 protein, formally known as Interleukin-1 receptor-associated kinase 2, is a pivotal player in cellular signaling pathways. It goes by various synonyms, including IRAK-2 and Pelle-like protein kinase. Belonging to the IRAK family, IRAK2 is a kinase, a type of enzyme that adds phosphate groups to other molecules, particularly proteins, as part of cellular signaling. Its structural characteristics and classification place it squarely in the realm of proteins essential for immune responses.
IRAK2 Biological Functions and Molecular Mechanisms
IRAK2 is a regulator of innate immunity. It operates downstream of Toll-like receptors (TLRs) and interleukin-1 receptors (IL-1Rs), pivotal components of the immune system. IRAK2 is a kinase that phosphorylates target proteins, setting off a cascade of molecular events.
The biological functions of IRAK2 are diverse and critical. It plays a crucial role in mediating the cellular response to bacterial and viral infections. By modulating the expression of inflammatory genes, IRAK2 ensures a harmonious immune response, preventing either excessive inflammation or insufficient defense.
At the molecular level, IRAK2 engages in intricate interactions with other proteins, forming complexes that act as molecular switches. These switches dictate whether a cell should activate an inflammatory response, undergo apoptosis, or maintain a state of equilibrium.
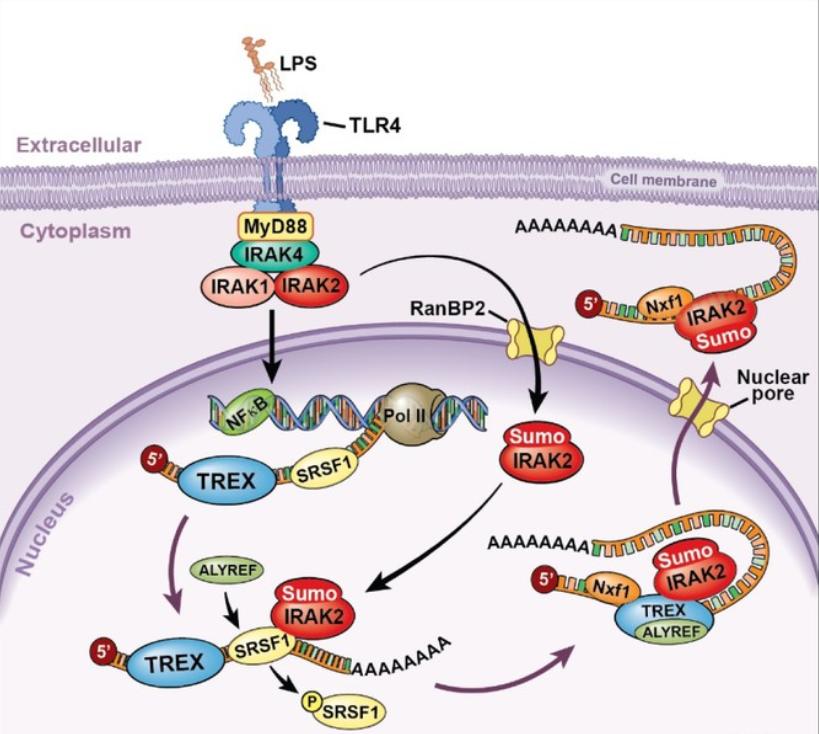
Figure 1. Model for the nuclear function of IRAK2. (Zhou H, 2017)
IRAK2 Related Signaling Pathway
The signal pathway orchestrated by IRAK2 is intricate and tightly regulated. Acting downstream of TLRs and IL-1Rs, IRAK2 forms complexes with other IRAK family members and accessory proteins. This culminates in the activation of transcription factors like NF-κB, driving the expression of genes involved in inflammation and immune responses.
Understanding the nuances of IRAK2-related pathways provides a roadmap for developing targeted interventions. Researchers are actively exploring the potential of IRAK2 inhibitors as a means to modulate immune responses and combat diseases characterized by aberrant inflammation.
IRAK2 Related Diseases
Understanding IRAK2's role in diseases provides a key to unraveling new therapeutic avenues. Dysregulation of IRAK2 has been implicated in various disorders, including autoimmune diseases and certain cancers. In rheumatoid arthritis and systemic lupus erythematosus, for instance, abnormal IRAK2 activity contributes to an overactive immune response, leading to chronic inflammation and tissue damage.
In the realm of cancer, IRAK2 has been implicated in promoting cell survival and resistance to apoptosis, fostering an environment conducive to tumor growth. Targeting IRAK2 pathways holds promise in developing novel therapeutic strategies against these diseases.
IRAK2's Applications in Biomedicine
IRAK2's versatility extends beyond its natural role in cellular signaling. In the biomedical arena, this protein has become a valuable tool in diagnostic development, vaccine design, and therapeutic interventions.
In diagnostics, the detection of IRAK2 levels can serve as a biomarker for certain diseases. Elevated IRAK2 expression may indicate an overactive immune response, guiding clinicians in tailoring treatment strategies.
Vaccine development also benefits from understanding IRAK2's role. Manipulating IRAK2 activity could enhance vaccine efficacy by fine-tuning immune responses, leading to more robust and targeted protection against pathogens.
In therapeutics, targeting IRAK2 holds promise for diseases where dysregulated immune responses contribute to pathology. Small molecule inhibitors and other modulators of IRAK2 activity are actively being explored for their potential in treating autoimmune diseases and certain cancers.
Recommended Products
| Cat.# | Product name | Species | Source (Host) | Tag |
|---|---|---|---|---|
| IRAK2-27503TH | Recombinant Human IRAK2 | Human | Insect Cell | N/A |
| IRAK2-2997H | Recombinant Human IRAK2 Protein (Val235-Ser521), His tagged | Human | E.coli | His |
| IRAK2-2753H | Recombinant Human IRAK2 protein, His-tagged | Human | E.coli | His |
| IRAK2-332H | Recombinant Human IRAK2, GST-tagged, Active | Human | Sf9 Insect Cell | GST |
| IRAK2-1417H | Active Recombinant Human IRAK2 (Active), GST-tagged | Human | Sf9 Insect Cell | GST |
| IRAK2-7069H | Recombinant Human IRAK2 protein, His-tagged | Human | E.coli | His |
| IRAK2-5053H | Recombinant Human IRAK2 Protein, GST-tagged | Human | Wheat Germ | GST |
| IRAK2-301417H | Recombinant Human IRAK2 protein, GST-tagged | Human | E.coli | GST |
| IRAK2-5729HF | Recombinant Full Length Human IRAK2 Protein, GST-tagged | Human | In Vitro Cell Free System | GST |
| Irak2-7070M | Recombinant Mouse Irak2 protein, His-tagged | Mouse | E.coli | His |
Reference
- Zhou H. IRAK2 directs stimulus-dependent nuclear export of inflammatory mRNAs. Elife 6. 2017.

