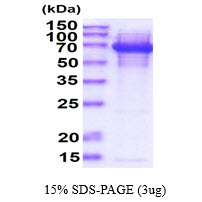
Bacterial Infections Targets

🧪 CdtB-01C
Source: E.coli
Species: Campylobacter jejuni
Tag: His
Conjugation:
Protein Length:
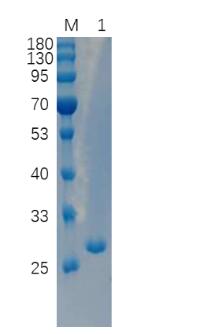
🧪 PLY-25S
Source: E.coli
Species: Streptococcus pneumoniae
Tag: His&SUMO
Conjugation:
Protein Length:
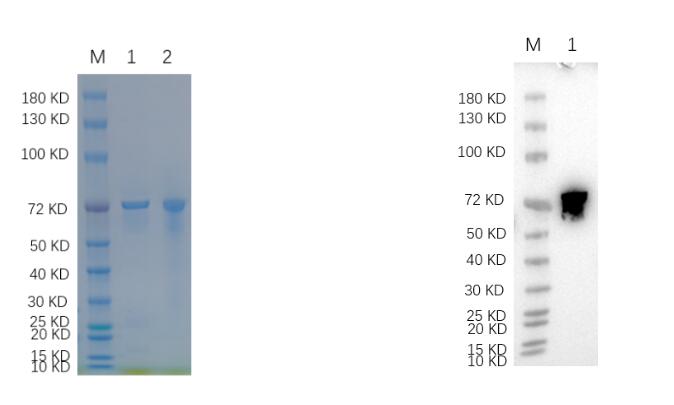
🧪 DnaK-1257H
Source: E.coli
Species: Mycobacterium Tuberculosis
Tag: Non
Conjugation:
Protein Length:

🧪 GroEL-1256M
Source: E.coli
Species: Mycobacterium Tuberculosis
Tag: Non
Conjugation:
Protein Length:

🧪 flaA-485L
Source: E.coli
Species: Listeria monocytogenes
Tag: Fc
Conjugation:
Protein Length: 1-287 a.a.

🧪 groEL2-33M
Source: E.coli
Species: Mycobacterium Tuberculosis
Tag: His
Conjugation:
Protein Length:

🧪 slo-01S
Source: Streptococcus Pyogenes
Species: Streptococcus pyogenes
Tag: Non
Conjugation:
Protein Length:

🧪 ospA-14B
Source: E.coli
Species: Borrelia Burgdorferi
Tag: His
Conjugation:
Protein Length:

Background
What are Bacterial Infections?
Bacterial infections are illnesses caused by bacteria, which are single-celled organisms that can invade the body and reproduce quickly. They can cause a range of symptoms depending on the type of bacteria and the part of the body affected. Some common symptoms include fever, chills, headaches, fatigue, and coughing.
Bacterial infections can spread through various means, including airborne transmission where diseases like tuberculosis spread through respiratory droplets, contaminated food or water, contact with contaminated objects, insect bites, and sexual contact. Different bacteria can cause different types of infections, and some common ones include Streptococcus, Staphylococcus, and E. coli.
Diagnosis of bacterial infections typically involves clinical assessments, laboratory tests, and imaging studies. Treatment usually involves antibiotics, which are effective in reducing symptoms. However, it's important to use antibiotics only when necessary and as prescribed to avoid antibiotic resistance.
To prevent bacterial infections, it's recommended to wash hands regularly, get vaccinated, practice proper food handling, drink clean water, and practice safe sex. While many bacterial infections are not serious and can be easily treated with antibiotics, infections that reach deep into the body, such as in the blood, heart, lungs, or brain, can be life-threatening.
What Causes Bacterial Infections?
Bacterial infections are caused by the invasion of harmful bacteria into the body where they can multiply and cause illness. These infections can affect any area of the body, including the skin, gut, lungs, heart, brain, and blood. Bacteria are everywhere, inside and on our body, in the air, water, soil, and the food we eat. While many bacteria are beneficial and perform vital functions such as aiding digestion and supporting our immune system, some can cause infections when they enter the body and multiply.
Bacteria can enter the body through various means, including:
- A cut or break in the skin
- Consumption of contaminated food or water
- Inhalation of droplets from an infected person
- Touching dirty surfaces followed by touching the eyes, nose, or mouth
- Transmission through blood and other bodily fluids
- Insect bites, such as ticks carrying Borrelia that causes Lyme disease
- Sexual contact, with infections like chlamydia, gonorrhea, and syphilis
What are the Types of Bacterial Infections?
Bacteria can cause many types of infections, depending on how you’re exposed and what part of your body it infects. Some common types of bacterial infections include:
Skin Infections
- Cellulitis: This is a skin infection that can occur after a break in the skin and is often caused by Staphylococcus aureus or Streptococcus pyogenes.
- Skin Abscesses: These are pus-filled pockets that can form under the skin, often caused by Staphylococcus aureus.
- Impetigo: A highly contagious superficial skin infection caused by Staphylococcus aureus or Streptococcus pyogenes, characterized by red sores on the skin.
- MRSA Infections: Methicillin-resistant Staphylococcus aureus (MRSA) is a type of staph bacteria that is resistant to many antibiotics and can cause skin infections and more serious conditions.
Respiratory Infections
- Pneumonia: Often caused by Streptococcus pneumoniae, pneumonia is an infection of the lungs that can cause cough, fever, and difficulty breathing.
- Strep Throat (Streptococcal Pharyngitis): Caused by Group A Streptococcus bacteria, it leads to a painful throat and fever.
- Tuberculosis (TB): Caused by Mycobacterium tuberculosis, TB primarily affects the lungs but can also affect other parts of the body.
- Tonsillitis: Inflammation of the tonsils, which can be caused by bacteria, particularly Streptococcus pyogenes.
Gastrointestinal Infections
- Food Poisoning: Caused by bacteria such as Salmonella, Campylobacter, and E. coli, food poisoning can lead to severe gastrointestinal symptoms.
- Gastritis and Peptic Ulcers: Helicobacter pylori, a type of bacteria, is associated with gastritis and peptic ulcers in the stomach and small intestine.
Genital Infections
- Chlamydia: While often grouped with sexually transmitted infection (STI), Chlamydia trachomatis is a bacterium that can cause infections in the reproductive system.
- Gonorrhea: Caused by Neisseria gonorrhoeae, gonorrhea is an STI that can affect both men and women.
- Syphilis: Caused by the bacterium Treponema pallidum, syphilis is an STI that can have long-term complications if not treated.
There are other diseases with site infections that are not clearly classified as follows:
- Urinary Tract Infections (UTIs): E. coli is a common cause of UTIs, which can affect the bladder, ureters, or kidneys and cause symptoms like painful urination and frequent urination.
- Tetanus: Caused by Clostridium tetani, this infection affects the nervous system and can cause muscle stiffness and spasms.
- Anthrax: Caused by Bacillus anthracis, anthrax is a rare but serious infection that can affect the skin, lungs, and gastrointestinal tract.
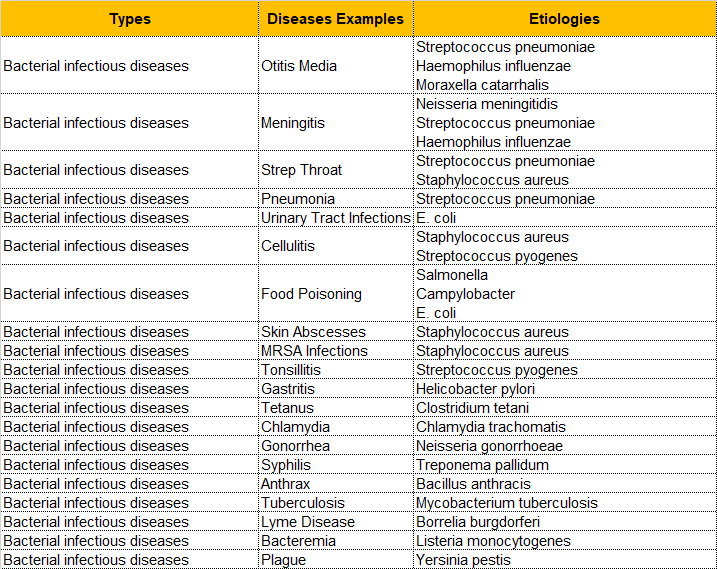
As for some common bacterial infections, we have mapped them to the bacteria that cause these diseases, and sorted out a table that can be referred to. The results are as follows:
Symptoms of Bacterial Infections
Symptoms depend on what part of the body is affected by the bacterial infection. Common symptoms of bacterial infections include:
- Fever.
- Chills.
- Fatigue (tiredness).
- Headache.

Additional symptoms can include:
- Bacterial skin infections: Symptoms may include a rash, pus-filled blisters, nodules, redness, swelling, or pain.
- Bacterial respiratory infections: Symptoms may include a cough, congestion, chest tightness, difficulty breathing, fever, sinusitis, or a sore throat.
- Gastrointestinal bacterial infections (such as from food contaminated with bacteria): Symptoms may include abdominal pain or cramps, nausea, vomiting, diarrhea, or fever.
- Sexually transmitted bacterial infections: Some are symptomless, but symptoms may include an unusual or strong smelling vaginal or penile discharge, skin changes in the genital region, pain, or infertility.
- Bacterial urinary tract infection: Symptoms may include pain while urinating, increased frequency of urination or an urgent need to urinate, pain around the pubic or kidney area.
- Bacterial meningitis (a bacterial infection of the outer layer of the brain): Symptoms may include a severe headache, a rash, a stiff neck, nausea, vomiting, confusion, or light sensitivity.
How to Treat Bacterial Infections?
The first thing you need to determine is whether it is a bacterial infection. Bacterial culture and drug susceptibility testing are used to identify the types of pathogens and select appropriate antimicrobial agents. Standardized specimen collection and timely delivery are essential to improve the detection rate. According to the drug sensitivity report, effective antibacterial drugs were selected for treatment. Treatment may include:
- Oral antibiotics
- Topical antibiotics
- Pain relievers
- Decongestants
- Probiotics
- Drainage

Bacterial infections can last for days to weeks but often go away on their own without antibiotics. That said, you may need prescription antibiotics if your body is unable to fight off a bacterial infection.
If you have symptoms like fever, pain, swelling, coughing, or dehydration, your healthcare provider may suggest anti-inflammatory medication. In addition to antimicrobials, symptomatic treatment may also be required, such as fever reduction, fluid rehydration, nutritional support, etc. For some infections, such as abscesses, surgical drainage or other local treatment may be required.
Case Study
Case Study 1: Recombinant Human TLR9 protein (TLR9-48H)
One of the changes brought about by Wallerian degeneration distal to nerve injury is disintegration of axonal mitochondria and consequent leakage of mitochondrial DNA (mtDNA)-the natural ligand for the toll-like receptor 9 (TLR9). RT-PCR and immunohistochemical or Western blot analyses were used to detect TLR9 mRNA and protein respectively in the lumbar (L4-L5) and cervical (C7-C8) dorsal root ganglia (DRG) ipsilateral and contralateral to a sterile unilateral sciatic nerve compression or transection. And researchers perfomed Double Immunostaining with rhTLR9 to detect cellular and intracellular TLR9 localization. The unilateral sciatic nerve lesions led to bilateral increases in levels of both TLR9 mRNA and protein not only in the lumbar but also in the remote cervical DRG compared with naive or sham-operated controls. This upregulation of TLR9 was linked to activation of the Nuclear Factor kappa B (NFκB) and nuclear translocation of the Signal Transducer and Activator of Transcription 3 (STAT3).
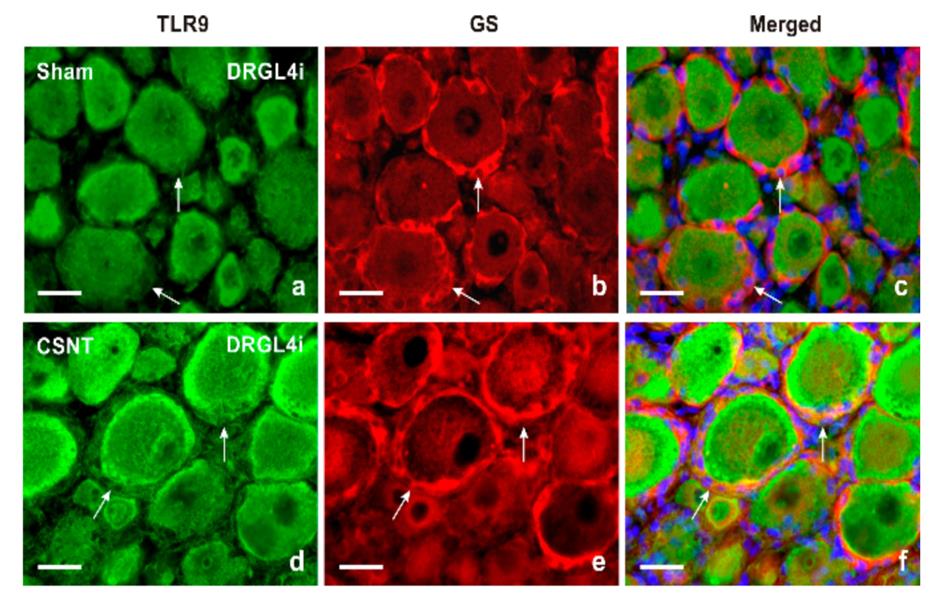
Fig1. Double immunofluorescence staining for TLR9 and GS as a marker of satellite glial cells (SGCs). (Petr Dubový, 2021)
Case Study 2: Recombinant Human Tumor Necrosis Factor alpha-1a (TNF-24H)
Most lung transplants are obtained from brain-dead donors. Administering methylprednisolone 60 min after inducing brain death in rats has been shown to modulate pulmonary inflammatory activity. This study was to evaluate the effects of methylprednisolone on transplanted rat lungs from donors treated 60 min after brain death. Twelve Wistar rats were anesthetized, and brain death was induced. They were given either the drug or a placebo. And the hemodynamic and blood gas parameters, histological score, lung tissue levels of thiobarbituric acid-reactive substances, level of superoxide dismutase, level of tumor necrosis factor-alpha, and level of interleukin-1 beta were evaluated. rhTNF-α was used as positive control in pulmonary TNF-α and IL-1β assay. After transplantation, a significant reduction in the levels of tumor necrosis factor-alpha and IL-1β was observed in the group that received methylprednisolone.
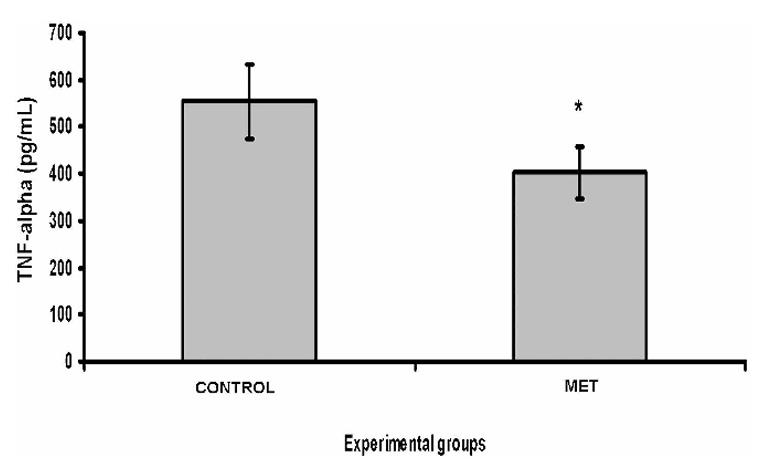
Fig2. Pulmonary tumor necrosis factor-alpha assay. (Luiz Felipe Lopes Araujo, 2014)
Case Study 3: Recombinant Cynomolgus PDCD1 protein (PDCD1-5223C)
In human clinical trials, GITR agonist antibodies have shown limited therapeutic effect, which may be due to suboptimal receptor clustering-mediated signaling. To overcome this potential limitation, here researchers show a bispecific molecule consisting of an anti-PD-1 antibody fused with a multimeric GITR ligand (GITR-L) that induces PD-1-dependent and FcγR-independent GITR clustering. The anti-PD-1-GITR-L bispecific is a PD-1-directed GITR-L construct that demonstrated dose-dependent, immunologically driven tumor growth inhibition in syngeneic, genetically engineered and xenograft humanized mouse tumor models, with a dose-dependent correlation between target saturation and Ki67 and TIGIT upregulation on memory T cells. Anti-PD-1-GITR-L thus represents a bispecific approach to directing GITR agonism for cancer immunotherapy.
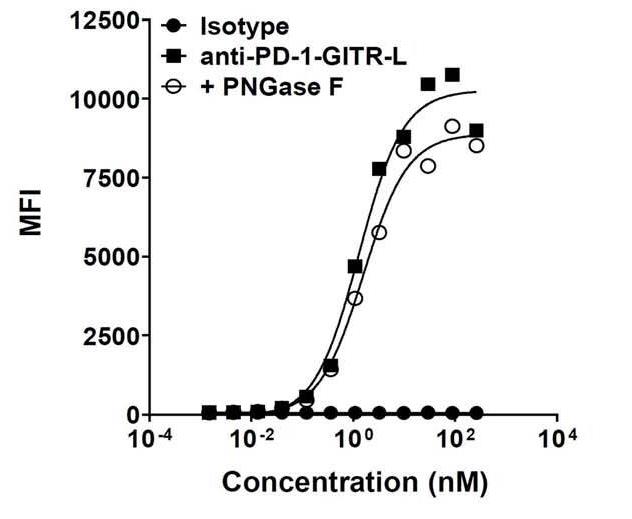
Fig3. Binding of de-glycosylated variants of anti-huPD-1-huGITR-L to human PD-1. (Sarah Chan, 2022)
Related Resources
Bacterial infectious diseases are a class of diseases caused by bacteria that can be transmitted through air, water, food, contact and many other ways. With the development of medicine, many bacterial infectious diseases have been effectively controlled through vaccination and antibiotic treatment. However, the problem of new strains of bacteria and drug resistance still requires us to remain vigilant and constantly research and develop new control strategies. With our deeper understanding of bacterial infectious diseases and advances in technology, we have the ability to better prevent and treat these diseases.