Protein Biotinylation
Protein biotinylation has become an indispensable tool in modern biochemical and biomedical research, enabling precise detection, immobilization, interaction analysis, and functional assays across a broad range of applications. With over a decade of specialized expertise, Creative BioMart delivers comprehensive custom biotinylation services tailored to the unique needs of academic laboratories, biotechnology enterprises, and pharmaceutical companies. Our platform supports both in vitro and in vivo labeling strategies designed to preserve protein integrity, ensure high labeling efficiency, and provide compatibility with downstream assays. From method selection to final quality assessment, we offer a complete, reliable, and application-oriented solution to accelerate your discovery and development projects.

Background: Understanding Protein Biotinylation
Biotinylation refers to the process of covalently attaching biotin molecules to proteins, peptides, or other biologically relevant targets. Owing to the extraordinarily high affinity of biotin for streptavidin and avidin, biotinylated biomolecules are widely used in applications involving immobilization, purification, detection, and molecular interaction studies. Their versatility extends into ELISA, Western blotting, protein pull-downs, surface plasmon resonance (SPR), flow cytometry, imaging, and targeted delivery systems.
Two primary strategies exist for biotinylating proteins:
- In vitro chemical or enzymatic biotinylation, performed after protein expression and purification. This method offers broad applicability, enabling modification of virtually any protein with accessible functional groups—including amines, sulfhydryls, carboxyls, and phosphates. Chemical biotinylation reagents with variable linker lengths or photoreactive groups can be selected to minimize steric hindrance and match specific technical requirements.
- In vivo biotinylation, achieved by genetically fusing a biotin acceptor peptide (BtAP) to the protein of interest and co-expressing it with biotin ligase BirA. This approach ensures site-specific, uniform, and stoichiometric biotinylation, providing extremely controlled and homogeneous labeled protein products.
Creative BioMart has built a robust, fully optimized biotinylation service platform covering both approaches. Whether the goal is to generate highly consistent conjugates for structural studies or to create large-scale labeled proteins for high-throughput screening, our scientists offer the expertise, reagents, vector constructs, expression systems, and quality control needed to guarantee reliability and reproducibility.
What We Offer: Protein Biotinylation In Vitro and In Vivo
Service Workflow

End-to-end Protein Biotinylation Solutions
Creative BioMart delivers end-to-end protein biotinylation solutions. Our service portfolio includes—but is not limited to—the following:
-
Custom Protein Biotinylation
We provide biotinylation for any protein of interest, regardless of molecular size, structural complexity, or biological origin. Multiple chemical and enzymatic strategies are available to support different downstream applications.
-
Post-Modification Purification
Following biotinylation, proteins are rigorously purified to remove unreacted biotin, excess reagents, or unwanted by-products. Purification methods include affinity chromatography, size-exclusion chromatography, ultrafiltration, and HPLC, depending on the protein properties and customer specifications.
-
Degree of Biotinylation Quantification
We offer precise measurement of the biotinylation level (biotin-to-protein molar ratio) to ensure labeling efficiency and lot-to-lot consistency.
-
Functional Evaluation of Biotinylated Proteins
Our team performs activity tests to verify that the biotinylation process has not disrupted protein structure or biological function. Both in vitro and in vivo assays are available depending on protein type.
Our In Vitro and In Vivo Platforms
|
|
In Vitro Biotinylation Platform 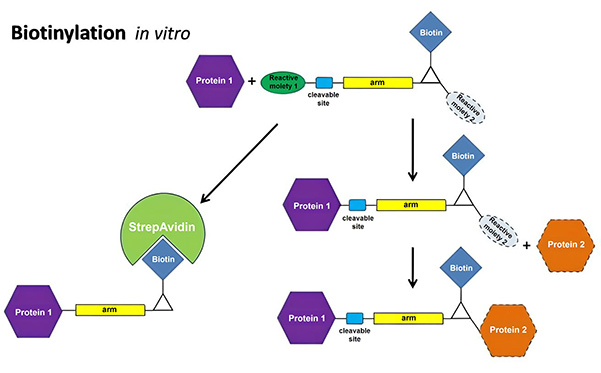 |
In Vivo Biotinylation Platform 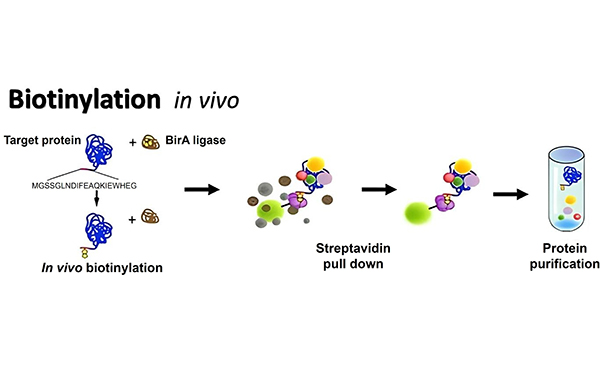 |
|
Services |
Our chemical and enzymatic in vitro labeling platform provides:
|
We offer E. coli - and mammalian-based in vivo biotinylation systems featuring:
|
|
Supported Approaches/Systems |
Our in vitro platform supports various chemical and enzymatic approaches:
|
Creative BioMart offers highly optimized in vivo biotinylation systems:
Mammalian Cell Biotinylation
|
|
Quality Standards |
All projects follow strict internal SOPs to ensure:
|
|
What Sets Us Apart
- More than 10 Years of Specialized Experience: Our extensive experience with protein biotinylation allows us to address routine, advanced, and highly specialized labeling challenges with technical confidence and proven methodologies.
- Comprehensive Biotinylation Options: We offer one of the industry's most complete portfolios, including amine-, sulfhydryl-, carboxyl-, and phosphate-reactive chemistries, long-linker biotin, photoreactive reagents, and enzymatic and genetic labeling systems.
- Preserved Protein Bioactivity: Every biotinylation reaction is carefully optimized to protect protein structure and function, supported by configurable analytical and functional activity testing.
- Highly Efficient In Vivo Biotinylation Platforms: Our proprietary BirA co-expression vectors and optimized hosts provide exceptional efficiency and reproducibility, enabling stoichiometric, site-specific biotinylation unmatched by standard systems.
- Tailored Solutions and Scalable Production: Whether you need microgram-scale pilot studies or multi-gram production for screening and manufacturing, we deliver fully customized workflows designed around your experimental goals.
- Rigorous Quality Control and Transparent Reporting: Each project comes with detailed documentation, QC data, reagent information, and additional optional analyses. You receive exactly what you need to confidently integrate the biotinylated protein into downstream workflows.
Case Studies and Practical Insights
Case 1: Quick evaluation of kinase inhibitors by SPR using single-site specifically biotinylated kinases
Kitagawa et al., 2014. doi:10.1177/1087057113506051
In evaluating kinase inhibitors, accurate kinetic and equilibrium parameters such as association/dissociation rates, K D, and IC50 are critical. Conventional SPR assays are often complicated by protein immobilization-induced conformational changes and heterogeneous orientations, as well as being time-consuming. To address these issues, single-site specifically biotinylated kinases were used with a multichannel SPR device, enabling precise kinetic measurements for staurosporine, dasatinib, sunitinib, and lapatinib across six kinases. Notably, the slow off-rates of lapatinib from EGFR and dasatinib from BTK and CSF1R were confirmed. IC50 values were further determined by activity-based assays, providing integrated physicochemical and biochemical insights into compound behavior.
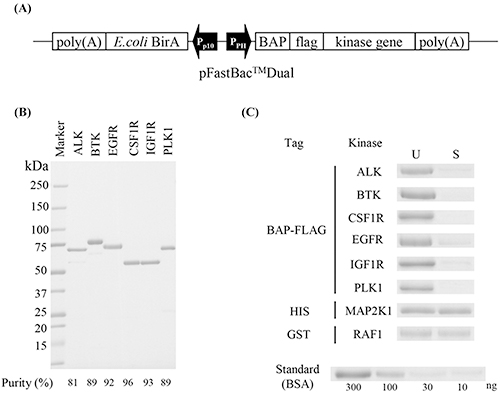
Figure 1. Single-site biotinylated kinases. (A) Construction; (B) Purification; (C) Biotinylation efficiency. (Kitagawa et al., 2014)
Case 2: Generation of peptides for highly efficient proximity utilizing site-specific biotinylation in cells
Kulyyassov et al., 2022. doi:10.3390/life12020300
Protein tags are widely used for purification, detection, and functional analysis, including in vivo labeling to study protein–protein interactions (PPI). Conventional AviTag biotinylation, while efficient, is too rapid for precise PPI analysis. To address this, new biotin acceptor peptides, BAP1070 and BAP1108, were developed using modular assembly, DNA sequencing, transient protein expression, and Western blot validation. These tags enable the Proximity Utilizing Biotinylation (PUB) method,coexpressing BAP-X and BirA-Y in mammalian cells, allowing detection of interacting proteins with low background. Over 100 constructs of transcription factors, histones, and nuclear proteins were successfully generated, demonstrating the method’s versatility and reliability in proteomics studies.
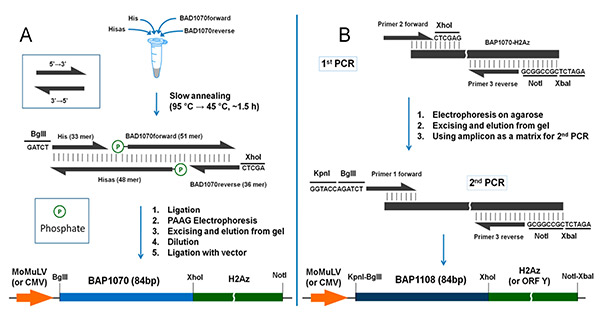
Figure 2. Workplans (A,B) for the generation of BAP1070 and BAP1108 fragments and subcloning into vectors for use as site-specific biotinylation targets in the PUB method. (Kulyyassov et al., 2022)
Customer Feedback on Protein Biotinylation
“We approached Creative BioMart for help producing site-specific biotinylated receptor constructs for a series of SPR kinetic assays. Our internal attempts using chemical biotinylation introduced too much heterogeneity, compromising data quality. Their BtAP–BirA in vivo system delivered perfectly uniform, stoichiometrically labeled receptors in record time. The resulting SPR chips exhibited exceptional stability and produced the cleanest association/dissociation curves we’ve ever collected. Their technical communication was clear, fast, and always insightful.”
— Senior Scientist, Biotherapeutics R&D | Global Pharmaceutical Company
“We needed kilogram-level production of a biotinylated enzyme for a diagnostic kit launch. Creative BioMart handled both expression and in vitro biotinylation using a long-chain linker that preserved full enzyme activity—something other vendors struggled to achieve at scale. They also performed stringent activity assays and provided batch-to-batch comparison data without us even asking. The final product bound our streptavidin-coated magnetic beads with remarkable consistency and supported automated workflows seamlessly. Their ability to scale from a milligram pilot batch to full production without losing performance saved us enormous time and budget.”
— Director of Assay Development | Mid-Size Biotechnology Company
“Our lab required photoreactive biotin labeling of a multi-pass membrane protein to capture transient interaction partners under native conditions. It was a technically messy project with low-expression constructs and fragile complexes. Creative BioMart optimized both solubilization and photolabeling conditions, then delivered biotinylated samples that worked perfectly with our pull-down and MS pipeline.”
— Principal Investigator | University Structural Biology Laboratory
“We engaged Creative BioMart to produce biotinylated versions of a heavily glycosylated secreted cytokine for flow-cytometry–based receptor profiling. They recommended a mammalian in vivo biotinylation strategy to ensure correct folding and glycoform presentation—an excellent call. The biotinylated cytokine showed outstanding binding fidelity across all receptor variants we screened, even at low picomolar concentrations. Their team anticipated potential stability issues and provided formulation suggestions that extended shelf life by months. Their scientific thoroughness and rapid turnaround made them one of the most reliable partners we’ve worked with.”
— Head of Protein Engineering | Immunology Startup
FAQs About Protein Biotinylation Services
-
Q: Can you biotinylate any protein, even if it has complex structure or modifications?
A: Yes. We support biotinylation for virtually any protein, including enzymes, receptors, antibodies, membrane proteins, and glycosylated or multi-domain targets. Our platform includes both in vitro chemical/enzymatic approaches and in vivo BtAP–BirA systems, allowing us to choose the most suitable method based on your protein’s characteristics. For proteins requiring native folding or post-translational modifications, we also offer mammalian cell–based in vivo biotinylation to ensure structural integrity and proper function. -
Q: How do you ensure that biotinylation does not affect protein bioactivity?
A: Maintaining protein functionality is one of our top priorities. We carefully optimize reaction conditions—such as reagent ratios, buffer composition, and linker length—to minimize structural perturbation. Additionally, we provide post-labeling activity testing, binding assays, and functional QC to confirm that the biotinylated protein behaves as expected in your downstream application. -
Q: What makes your in vivo biotinylation system different from others?
A: Our in vivo platform features a highly optimized BtAP–BirA co-expression vector system and proprietary host strains, enabling near-100% stoichiometric, site-specific biotinylation. This dramatically reduces batch-to-batch variability and eliminates labeling heterogeneity typically observed with chemical methods. The result is superior performance in applications like SPR, receptor binding studies, and quantitative assays. -
Q: Do you offer long-linker or specialized biotinylation chemistries?
A: Absolutely. We provide a broad range of chemistries, including:- Long-chain spacer arm biotin to reduce steric hindrance
- Photoreactive biotin for membrane proteins or proteins with few accessible reactive groups
- Cleavable or reversible biotin for gentle elution from streptavidin
- Amine-, sulfhydryl-, carboxyl-, and phosphate-reactive biotin reagents
-
Q: What level of control do you offer over labeling efficiency?
A: We quantify the degree of biotinylation (biotin-to-protein ratio) for every project and can adjust conditions to achieve your preferred labeling density—whether you need a single controlled site or a higher labeling level for detection-driven assays. Each batch includes full QC documentation for traceability and confidence. -
Q: Do you offer purification after biotinylation?
A: Yes. After labeling, we perform thorough post-modification purification to remove excess reagents and ensure high sample purity. Depending on the protein and project goals, we use affinity chromatography, size exclusion chromatography, ultrafiltration, and HPLC. This ensures clean, ready-to-use biotinylated protein for your downstream applications. -
Q: What if my protein is difficult to express or purify?
A: No problem—our team can also handle upstream protein production in bacterial, yeast, insect, or mammalian systems. For challenging proteins, we optimize expression, solubility, and purification strategies before biotinylation. Many clients rely on us specifically because we specialize in complex, “non-standard” protein targets. -
Q: How fast is your typical turnaround time?
A: Turnaround time depends on the project scale and biotinylation method, but most in vitro biotinylation projects can be completed within days, while in vivo projects typically require 2–4 weeks. Our optimized workflows and extensive experience help us deliver high-quality results quickly without compromising precision. -
Q: How do I know which biotinylation strategy is best for my project?
A: You don’t have to decide alone. Our scientists evaluate your protein’s structure, application, stability requirements, and functional constraints to recommend the most suitable approach—chemical, enzymatic, or in vivo . We routinely help clients troubleshoot designs for SPR, pull-downs, imaging, flow cytometry, diagnostics, and more.
Other Resources
Related Services
- Enzyme Immobilization
- Protein Interaction Service
- Protein Expression and Purification Services
- Pull-Down Assays
- Surface Plasmon Resonance (SPR) Service
- High-Throughput Screening
- Protein Labeling
- Protein Bioconjugation Services
Related Products
References:
- Kitagawa D, Gouda M, Kirii Y. Quick evaluation of kinase inhibitors by surface plasmon resonance using single-site specifically biotinylated kinases. SLAS Discovery. 2014;19(3):453-461. doi:10.1177/1087057113506051
- Kulyyassov A, Ramankulov Y, Ogryzko V. Generation of peptides for highly efficient proximity utilizing site-specific biotinylation in cells Life. 2022;12(2):300. doi:10.3390/life12020300
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

