Dual-Targeted Theranostic Probes for Prostate Cancer: Combining PROTACs and Radionuclide Therapy
Prostate cancer treatment faces challenges such as off-target effects and resistance to monotherapies. This study introduces ¹⁷⁷Lu-P-A, a unimolecular theranostic probe integrating proteolysis-targeting chimeras (PROTACs) and targeted radionuclide therapy (TRT) to address these limitations.
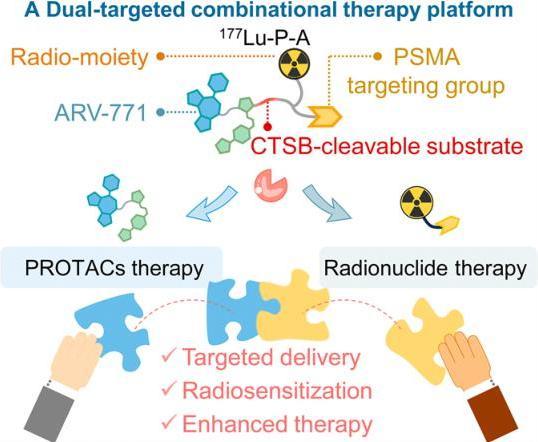
Key Innovations are:
- Dual Targeting Mechanism: The probe combines:
- A PSMA-targeting ligand for prostate-specific membrane antigen (PSMA)-mediated tumor accumulation.
- A CTSB-activatable PROTAC (ARV-771) that degrades bromodomain-containing protein 4 (BRD4) upon cleavage by cathepsin B (CTSB), an enzyme overexpressed in tumors.
- ¹⁷⁷Lu, a β-emitting radionuclide, for localized DNA damage.
- Synergistic Action:
- TRT delivers radiation directly to tumors, inducing DNA damage.
- PROTAC activation degrades BRD4, suppressing RAD51AP1 (a DNA repair protein) and enhancing radiosensitivity.
- Combined therapy amplifies apoptosis and reduces tumor resistance.
Key Findings are:
- In Vitro: ¹⁷⁷Lu-P-A showed 2.8-fold lower IC₅₀ compared to individual therapies. CTSB-mediated release of ARV-771 degraded BRD4 and downstream oncoproteins (e.g., c-Myc), while TRT caused DNA damage. Synergy was confirmed by reduced Bcl-2 (antiapoptotic) and elevated cleaved caspase-3 (proapoptotic).
- In Vivo: In LNCaP xenograft mice, combined therapy achieved 92.8% tumor growth inhibition vs. 60–70% for TRT or PROTAC alone. SPECT/CT imaging demonstrated high tumor uptake (14.9% ID/g) and rapid renal clearance (69% within 24 hours), minimizing systemic toxicity.
- Safety: No significant organ damage or serum toxicity was observed, highlighting biocompatibility.
Advantages Over Existing Therapies:
- Precision: PSMA targeting and CTSB activation ensure tumor-specific delivery, reducing off-target effects.
- Efficacy: The dual mechanism overcomes resistance seen with standalone PROTACs or TRT (e.g., ¹⁷⁷Lu-PSMA-617).
- Lower Radionuclide Dose: Synergy allows reduced radiation exposure without compromising efficacy.
Limitations and Future Directions:
- Clinical translation requires validation of safety and efficacy in humans.
- Scaling production and ensuring batch consistency remain challenges.
This study pioneers a unimolecular approach to combine protein degradation and radiation, offering a blueprint for next-generation cancer theranostics with enhanced precision and reduced toxicity.
Reference
- Zhang Y, Gu W, Chen W, Zhu J, Fan L, Zhang L, Zhao L, Miao Q. A Dual-Targeted Molecule for Disease-Activatable Proteolysis Targeting Chimeras and Targeted Radionuclide Therapy of Cancer. J Am Chem Soc. 2025 Mar 5;147(9):7897-7907. doi: 10.1021/jacs.4c18398. Epub 2025 Feb 24. PMID: 39989465.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

