pLYTAC-EndoTags based lysosome targeted degradation chimera
Targeted protein degradation has emerged as a transformative therapeutic strategy, but existing approaches face challenges like ligand competition and manufacturing complexity. The study presents a breakthrough: EndoTags, computationally designed proteins that hijack endocytosis to degrade extracellular and membrane-bound targets or amplify cellular signals. This innovation addresses critical gaps in precision medicine and synthetic biology.
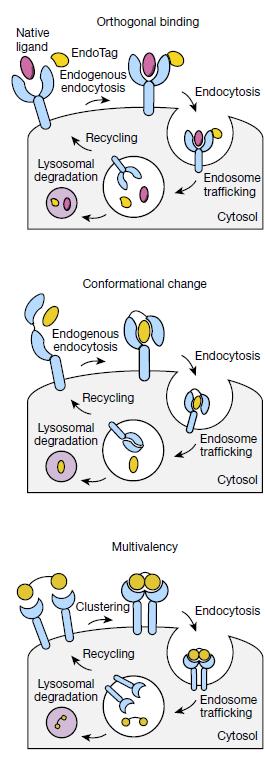
EndoTags exploit three receptor-specific mechanisms:
- Orthogonal Binding: For receptors like sortilin and TfR that naturally recycle, EndoTags bind non-overlapping sites, avoiding competition with endogenous ligands.
- Conformational Triggering: For IGF2R, EndoTags mimic ligand-induced structural changes, forcing receptor dimerization and internalization.
- Receptor Clustering: Multivalent EndoTags oligomerize ASGPR, enhancing uptake without chemical crosslinkers.
These strategies enable tissue-specific targeting-ASGPR for liver, TfR for brain-and bypass limitations of native ligand-dependent systems.
- Degradation of Pathogenic Proteins:
- EGFR: Fusing EGFR-binding domains to EndoTags reduced EGFR levels by 80% in cancer cells, dampening oncogenic signaling.
- PD-L1: Anti-PD-L1 antibodies fused to IGF2R-targeting EndoTags degraded PD-L1 in tumors, shrinking tumor volume and extending survival in mice.
- Autoantibodies: EndoTags linked to IgG-binding protein G cleared pathogenic antibodies, suggesting utility in autoimmune diseases.
- Soluble Protein Clearance: EndoTags effectively internalized synthetic and endogenous soluble proteins, such as IgG, demonstrating potential for treating antibody-driven disorders.
- Logic-Gated Degradation: Using Co-LOCKR systems, EndoTags degraded EGFR only in HER2⁺ cells, enhancing specificity and reducing off-target effects.
EndoTags amplified synthetic Notch receptor signaling by nearly 100-fold by promoting endosomal activation. This highlights their dual utility: degrading harmful proteins while enhancing therapeutic signaling pathways, such as in adoptive cell therapies.
Advantages Over Existing Methods
- Genetic Encodability: Unlike LYTACs requiring chemical conjugates, EndoTags are entirely protein-based, simplifying production and enabling secretion from engineered cells.
- Tissue Specificity: Receptor diversity allows tailored delivery (e.g., brain vs. liver).
- Reduced Off-Target Effects: Orthogonal binding minimizes interference with natural ligand functions.
While promising, challenges remain:
- Immunogenicity: Protein-based designs may trigger immune responses, necessitating further optimization.
- Long-Term Efficacy: Some constructs require continuous dosing; catalytic or recyclable variants could improve durability.
- Clinical Translation: Scaling production and validating safety in humans are critical next steps.
EndoTags represent a paradigm shift in targeted degradation and cellular engineering. Their modular design, compatibility with biologic therapeutics, and dual functionality in degradation and signaling position them as versatile tools for treating cancers, autoimmune diseases, and beyond. As computational protein design advances, EndoTags could unlock unprecedented precision in manipulating cellular processes, heralding a new era of customizable molecular therapeutics.
Reference
- Huang, B., Abedi, M., Ahn, G. et al. Designed endocytosis-inducing proteins degrade targets and amplify signals. Nature 638, 796–804 (2025). https://doi.org/10.1038/s41586-024-07948-2
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

