Other Proteins Involved in Regulating B Cell Activation
Related Symbol Search List
Immunology Background
Background
B cell activation is a fundamental process in the adaptive immune response, enabling B cells to recognize and respond to antigens. It involves a series of complex interactions and signaling events. Here's an introduction to B cell activation:
| Steps | Details |
|---|---|
| 1. B Cell Receptor (BCR) Recognition |
|
| 2. BCR Crosslinking and Signal Initiation |
|
| 3. BCR Signaling Pathways |
|
| 4. Co-stimulation and T Cell Help |
|
| 5. Internalization and Antigen Processing |
|
| 6. Antigen Presentation and T-B Cell Interaction |
|
| 7. B Cell Activation and Proliferation |
|
| 8. Differentiation into Effector Cells |
|
B cell activation is a tightly regulated process that ensures the generation of an effective immune response against antigens. It integrates signals from the BCR, co-stimulatory molecules, and T cell help, leading to B cell proliferation, differentiation, and the production of antibodies.
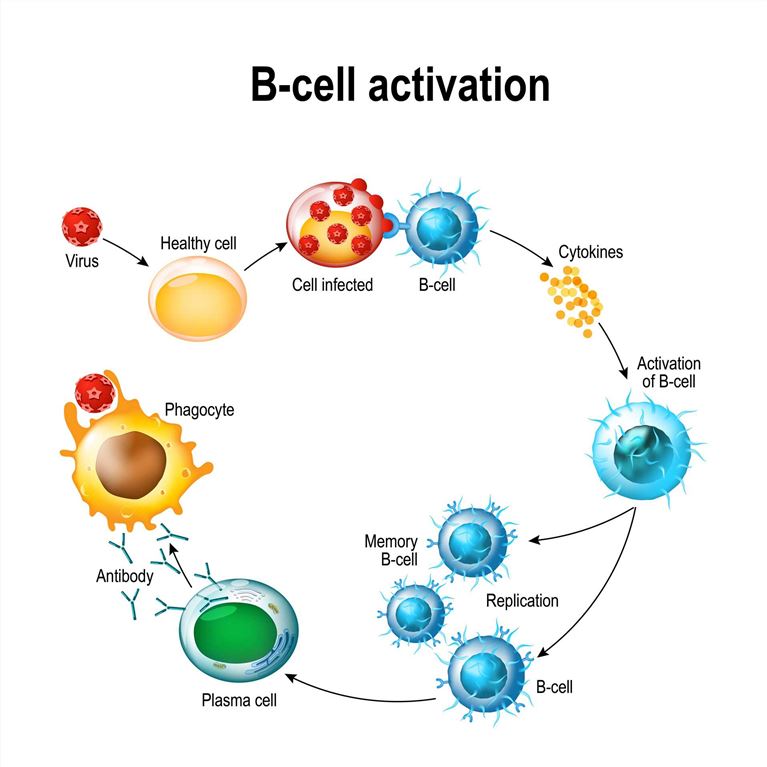
Molecules Involved in Regulating B Cell Activation
Several molecules play critical roles in regulating B cell activation. These proteins contribute to the signaling pathways, co-stimulation, and interactions necessary for efficient B cell activation. Here are some key proteins involved in the regulation of B cell activation:
| Key proteins | Functions |
|---|---|
| CR1 (Complement Receptor 1) |
|
| LAIR1 (Leukocyte-associated Immunoglobulin-like Receptor 1) |
|
| LILRA2 (Leukocyte Immunoglobulin-like Receptor Subfamily A Member 2) |
|
| LILRA3 (Leukocyte Immunoglobulin-like Receptor Subfamily A Member 3) |
|
| LILRB1 (Leukocyte Immunoglobulin-like Receptor Subfamily B Member 1) |
|
| ILT4 (Immunoglobulin-like Transcript 4) |
|
| LILRB3 (Leukocyte Immunoglobulin-like Receptor Subfamily B Member 3) |
|
| LILRB4 (Leukocyte Immunoglobulin-like Receptor Subfamily B Member 4) |
|
| SLAMF7 (Signaling Lymphocytic Activation Molecule Family Member 7) |
|
| VAV1 (Vav Guanine Nucleotide Exchange Factor 1) |
|
| B Cell Activating Factor (BAFF) and A Proliferation-Inducing Ligand (APRIL) |
|
Role of Proteins that Regulate B Cell Activation in Disease Development
Autoimmune Diseases
- Rheumatoid Arthritis (RA): Dysregulation of CR1 and LILRB1 has been associated with increased risk and severity of RA by affecting complement activation and immune responses.
Cancer
- Multiple Myeloma: SLAMF7 is highly expressed on plasma cells and serves as a therapeutic target in multiple myeloma. Monoclonal antibodies targeting SLAMF7, such as elotuzumab, have shown efficacy in treating multiple myeloma.
Infectious Diseases
- HIV/AIDS: VAV1 plays a role in B-cell receptor signaling and activation. Dysregulation of VAV1 has been implicated in B-cell dysfunction and impaired immune responses in HIV/AIDS.
Immunodeficiency Disorders
- Primary Immunodeficiency Disorders: Dysregulation of LILRA2 and LILRA3, members of the ILT/LIR family, may contribute to the development of primary immunodeficiency disorders by affecting immune responses and regulation.
Miscellaneous Diseases
- Fibrosis: LAIR1 is involved in the regulation of collagen production and tissue fibrosis. Dysregulation of LAIR1 has been implicated in fibrotic diseases, such as liver fibrosis and pulmonary fibrosis.
- Transplant Rejection: ILT4 is an inhibitory receptor expressed on immune cells, including B cells. It plays a role in immune tolerance and has been implicated in regulating transplant rejection responses.
- Inflammatory Disorders: Dysregulation of LILRB3 and LILRB4, inhibitory receptors, has been associated with inflammatory disorders such as inflammatory bowel disease (IBD) and autoimmune thyroiditis.
It's important to note that the roles of these proteins can vary depending on the specific disease context, and further research is needed to fully understand their mechanisms and therapeutic implications in each disease.
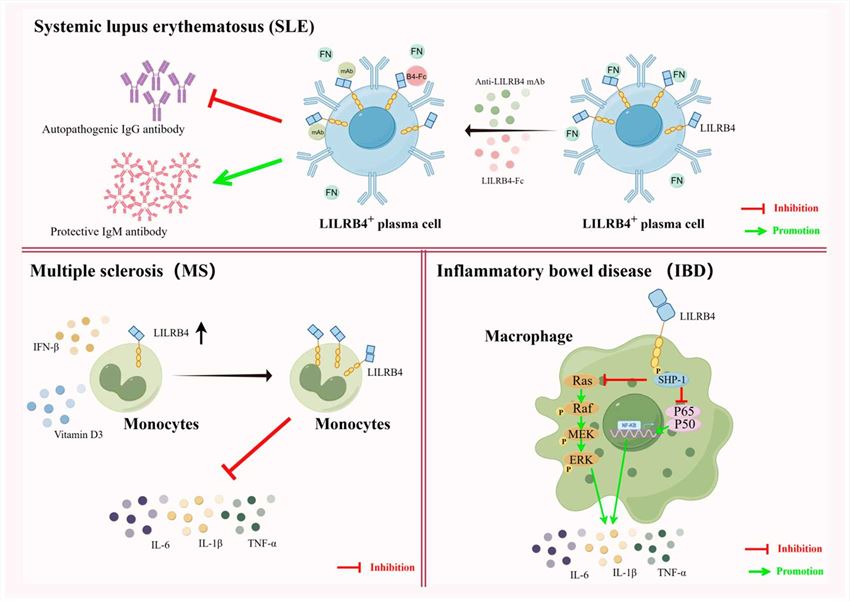 Fig.2 Diverse uses of LILRB4 in autoimmune diseases. (Xiang Z, et al., 2024)
Fig.2 Diverse uses of LILRB4 in autoimmune diseases. (Xiang Z, et al., 2024)Case Study
Case 1: O'Connell P, Blake MK, Godbehere S, Amalfitano A, Aldhamen YA. SLAMF7 modulates B cells and adaptive immunity to regulate susceptibility to CNS autoimmunity. J Neuroinflammation. 2022;19(1):241.
To evaluate how SLAMF7 intrinsically regulates B cell responses the authors cultured WT splenic B cells in vitro in the presence or absence of SLAMF7 receptor activation (via receptor cross-linking) and evaluated cell surface B cell activation markers via spectral cytometry. The authors found decreased autofluorescence (a proxy for lymphocyte activation) (D), MHC-II (E), GL7 (F), and PD-L1 expression (G) on SLAMF7-activated B cells compared to unstimulated B cells. Examination of soluble factors in supernatant of cultures of SLAMF7-stimulated B cells revealed decreased levels of Eotaxin (H), IL-17 (I), TNF⍺ (J), and CCL5 (K) in B cells with SLAMF7 activation compared to unstimulated cells, revealing an inhibitory role for SLAMF7 in B cells. While changes in some of these surface and soluble activation markers were minimal, together they suggest SLAMF7 signaling is capable of tempering B cell activation in a mild manner.
To determine what role SLAMF7 expression on B cells plays in regulating T cell responses, the authors set up an in vitro co-culture model consisting of T cells isolated from WT mice at peak EAE severity, combined with either WT or SLAMF7−/− B cells isolated from naïve mice, along with stimulation using MOG peptide. Using this model, the authors were able to assess the contribution of SLAMF7 expression on B cells to antigen-induced T cell proliferation and found that SLAMF7 expression on B cells restrains antigen-induced CD8+ T cell proliferation (L). Similarly, SLAMF7 expression on B cells also decreased antigen-induced PD-1 expression on CD8+ T cells (M) and CD69 expression on CD8+ T cells (N) (no significant changes found in CD4+ T cell phenotypes. Fittingly, the authors also found that SLAMF7−/− B cells express more CD80 compared to WT B cells (O).
Together, these results suggest that in the absence of SLAMF7, B cells are more prone to activation and preferentially induce CD8+ T cell activation, possibly via MHC-II, PD-L1, CD80, and/or direct SLAMF7 ligation mechanisms.
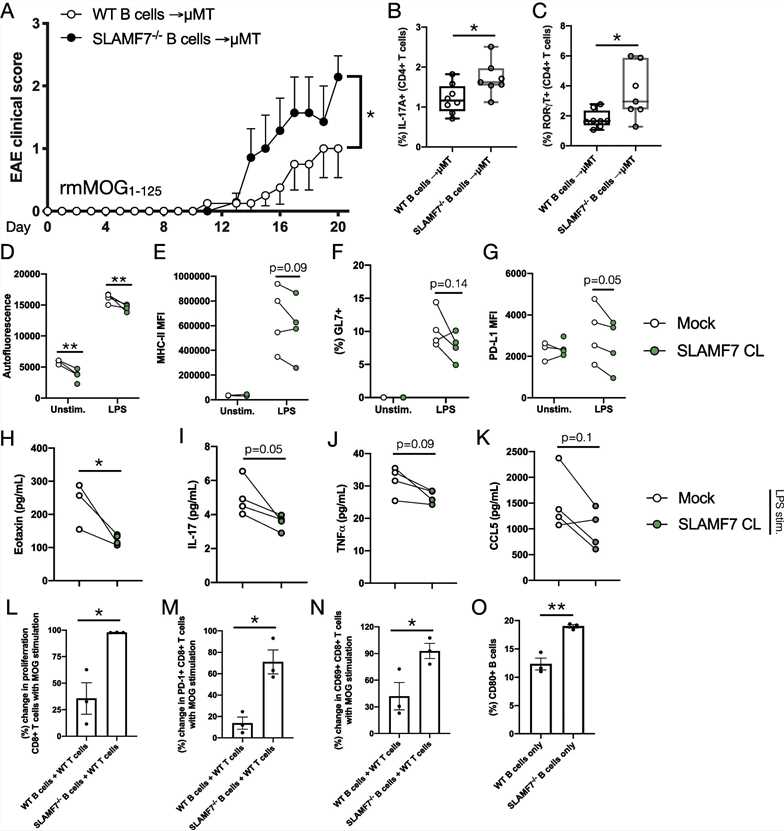 Fig.1 SLAMF7 modulates B cells and adaptive immunity to regulate susceptibility to CNS autoimmunity.
Fig.1 SLAMF7 modulates B cells and adaptive immunity to regulate susceptibility to CNS autoimmunity.References
- Xiang Z, Yin X, Wei L, Peng M, Zhu Q, Lu X, Guo J, Zhang J, Li X, Zou Y. LILRB4 Checkpoint for Immunotherapy: Structure, Mechanism and Disease Targets. Biomolecules. 2024; 14(2):187.
- Shlomchik MJ. Sites and Stages of Autoreactive B Cell Activation and Regulation. Immunity. 2008;28(1):18-28.
- Doherty DG, Melo AM, Moreno-Olivera A, Solomos AC. Activation and Regulation of B Cell Responses by Invariant Natural Killer T Cells. Front Immunol. 2018;9:1360.

