IVD of Apple Allergy
🧪 MALD1-1273A
Source: E.coli
Species: Apple
Tag: His&SUMO
Conjugation:
Protein Length: 2-159 a.a.
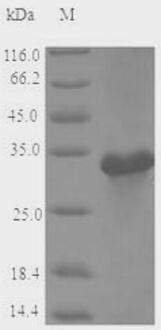
🧪 MALD1-1992A
Source: E.coli
Species: Apple
Tag: His&SUMO
Conjugation:
Protein Length: 2-159 aa
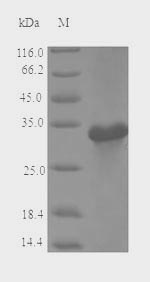
🧪 MALD3-1999A
Source: E.coli
Species: Apple
Tag: His&SUMO
Conjugation:
Protein Length: 25-115 aa
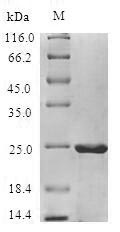
🧪 MALD1-2071M
Source: Yeast
Species: Malus Domestica
Tag: His
Conjugation:
Protein Length: 2-159 aa

🧪 Mald1-01H
Source: E.coli
Species: Malus Domestica
Tag: His
Conjugation:
Protein Length: 180
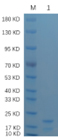
🧪 Mald2-01H
Source: E.coli
Species: Malus Domestica
Tag: His
Conjugation:
Protein Length: 243
🧪 Mald3-01H
Source: E.coli
Species: Malus Domestica
Tag: His
Conjugation:
Protein Length: 112
🧪 Mald4-01H
Source: E.coli
Species: Malus Domestica
Tag: His
Conjugation:
Protein Length: 151

🧪 LOC103436360-1616M
Source: E.coli
Species: Apple
Tag: His
Conjugation:
Protein Length: Ser72-Lys539

🧪 MALD1-5687A
Source: E.coli
Species: Apple
Tag: His
Conjugation:
Protein Length: Met1-Asn159

Background
Apple is a fruit rich in nutrients, dietary fiber, and water content, but some people will develop allergic symptoms after eating it. For patients who are allergic to apples, their immune systems do not recognize proteins such as Mal d 1, Mal d 2, and Mal d 3. Such allergenic proteins enter the body bind to IgE antibodies and promote the release of histamine, leading to diarrhea and vomiting. In addition, apples contain a large amount of fruit acids and vitamins, which combine with the tissues in the human body to form different chemicals to further stimulate the immune system and induce allergic reactions.

Causes and Symptoms
Causes: Apple allergy is often a result of oral allergy syndrome (OAS), which is also known as pollen-food syndrome. This occurs due to cross-reactivity between the proteins in apples and the proteins in certain pollens (like birch pollen).
Symptoms: Symptoms usually appear shortly after consuming raw apples. They include:
- Itching or swelling of the mouth, lips, and throat
- Tingling sensation in the mouth
- In more severe cases, symptoms can include hives, gastrointestinal issues, and even anaphylaxis (although this is rare).
Cross-Reactive Fruits
If you have an apple allergy, particularly one tied to birch pollen, you may also be allergic to other fruits and vegetables whose proteins resemble those in birch pollen. These include:
- Pears
- Peaches
- Plums
- Cherries
- Carrots
- Celery
- Potatoes
- Hazelnuts
Foods to Avoid
- Raw apples and products containing raw apples, such as apple salads or raw apple slices.
- Apple juice and apple cider can sometimes cause reactions if they contain raw apple ingredients.
- Baked or cooked apple products generally have lower allergenicity, but it's best to confirm with an allergist if they are safe for you.
Main Steps of IVD for Apple Allergy
- Skin Prick Test
Prick or inject apple extract into the skin and observe the skin's reaction to determine the allergic status.
- Serum IgE Antibody Test
In serological testing, apple allergy can usually be diagnosed if the patient has high levels of specific IgE antibodies.
- Oral Food Challenge (OFC)
Eat apples or related products and observe whether the patient has an allergic reaction to determine allergy.
Creative BioMart provides high-quality recombinant apple allergen protein used for IVD, including ELISA, lateral flow assay, western blot, and other immunoassays.
Highlights of Our Products
- Higher safety and repeatability. Genetic engineering technology allows it to be produced without non-specific allergens.
- High purity. No contamination from exogenous toxic substances and pathogenic microorganisms.
- High specificity. The molecular structure and biological activity of recombinant allergens are similar to natural allergens and can effectively stimulate immune responses in allergic patients.
- Easy to scale up production.
Our Outstanding Advantages
- A rich variety of IVD products to meet the different needs of customers and provide customers with comprehensive scientific research support.
- Strong technical team, advanced scientific research equipment, and technology that can provide high-quality services.
- Focus on delivering high-quality services to customers, providing timely and precise IVD protein services.
In addition, Creative BioMart also offers a series of allergen proteins and protein-related services to provide customers with high-quality, low-cost active recombinant proteins to meet different needs and assist in preclinical drug development.
Case Study
Case 1: Nothegger B, Reider N, Covaciu CE, Cova V, Ahammer L, Eidelpes R, Unterhauser J, Platzgummer S, Tollinger M, Letschka T, Eisendle K. Allergen-specific immunotherapy with apples: selected cultivars could be a promising tool for birch pollen allergy. J Eur Acad Dermatol Venereol. 2020 Jun;34(6):1286-1292. doi: 10.1111/jdv.16201. Epub 2020 Feb 16. PMID: 31953891; PMCID: PMC7318684.
As continuous apple consumption might also mitigate the inhalational allergy, this study aimed to uncover apple cultivars suitable for treatment of birch pollen rhinoconjunctivitis and apple allergy in a controlled and established dosage.
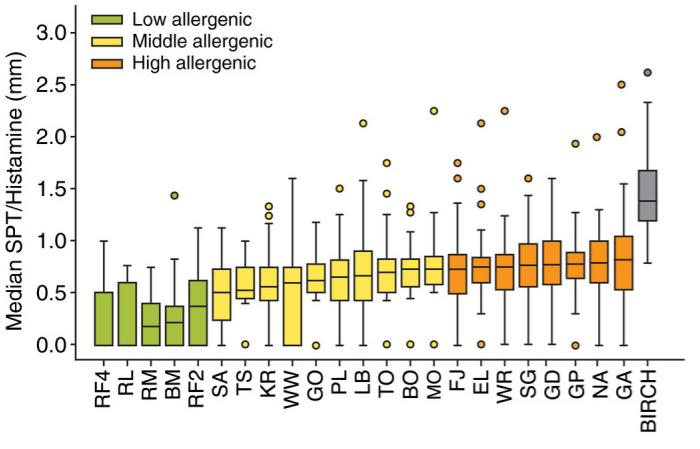 Fig2. Apple prick‐to‐prick test (SPT) with 23 apple cultivars including birch, median SPT of flesh–peel–stalk/histamine (mm) [HEP‐Index]. Twenty‐one cultivars were tested in Austria and Italy (n = 52), EL and GP solely in Austria (n = 31). The apples are ranked by increasing allergenicity and divided into three groups (low, middle and high allergenic) based on pairwise comparison using the Friedman test with Dunns post‐test, P < 0.05. The low allergenic group (HEP‐Index < 0.5) showed no intragroup differences and was significantly different from the high allergenic group (HEP‐Index > 0.7) which also displayed no statistically significant intragroup differences.
Fig2. Apple prick‐to‐prick test (SPT) with 23 apple cultivars including birch, median SPT of flesh–peel–stalk/histamine (mm) [HEP‐Index]. Twenty‐one cultivars were tested in Austria and Italy (n = 52), EL and GP solely in Austria (n = 31). The apples are ranked by increasing allergenicity and divided into three groups (low, middle and high allergenic) based on pairwise comparison using the Friedman test with Dunns post‐test, P < 0.05. The low allergenic group (HEP‐Index < 0.5) showed no intragroup differences and was significantly different from the high allergenic group (HEP‐Index > 0.7) which also displayed no statistically significant intragroup differences.Case 2: Haka J, Niemi MH, Iljin K, Reddy VS, Takkinen K, Laukkanen ML. Isolation of Mal d 1 and Api g 1 - specific recombinant antibodies from mouse IgG Fab fragment libraries - Mal d 1-specific antibody exhibits cross-reactivity against Bet v 1. BMC Biotechnol. 2015 May 27;15:34. doi: 10.1186/s12896-015-0157-5. PMID: 26013405; PMCID: PMC4446070.
Individuals with certain pollen allergies may also suffer from a sensitisation to proteins in the food products. As an example a person sensitised to the major birch pollen allergen, Bet v 1, is often sensitised to its homologues, such as the major allergens of apple, Mal d 1, and celery, Api g 1, as well. Development of tools for the reliable, sensitive and quick detection of allergens present in various food products is essential for allergic persons to prevent the consumption of substances causing mild and even life-threatening immune responses.
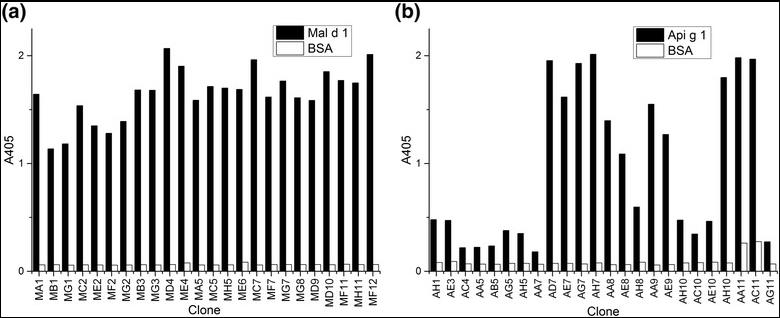 Fig3. Screening of the rMal d 1 and rApi g 1 -specific antibody phages by ELISA. The specific binding of the individual anti-rMal d 1 (a) and anti-rApi g 1 (b) Fab fragment phage clones against their target allergen and BSA background has been analysed.
Fig3. Screening of the rMal d 1 and rApi g 1 -specific antibody phages by ELISA. The specific binding of the individual anti-rMal d 1 (a) and anti-rApi g 1 (b) Fab fragment phage clones against their target allergen and BSA background has been analysed.




