What is F9 Protein
In the intricate tapestry of human biology, proteins play a crucial role in maintaining the delicate balance required for proper functioning. Among these, the F9 protein, also known as Factor IX, stands out as a key player in blood coagulation.
F9, a pivotal player in the intricate choreography of blood coagulation, is a serine protease encoded by the F9 gene on the X chromosome. This protein, also known as Christmas Factor, is integral to the intrinsic pathway of the blood clotting cascade. Structurally, F9 belongs to the vitamin K-dependent clotting factor family, necessitating the presence of vitamin K for its activation.
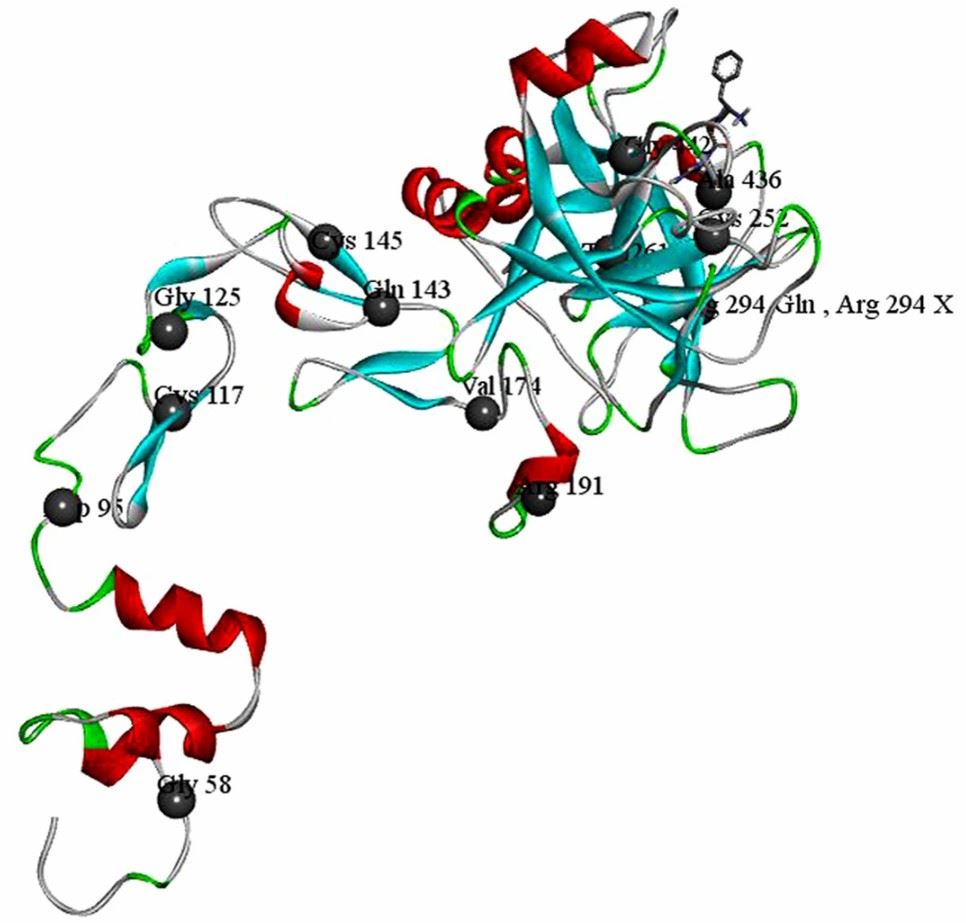 Figure 1. Crystal structure of factor (F) IXa and locations of the missense mutations. (Wang, QY., et al. 2016)
Figure 1. Crystal structure of factor (F) IXa and locations of the missense mutations. (Wang, QY., et al. 2016)The Function of F9 Protein
At the heart of blood coagulation, F9's primary function is to act as a co-factor in the activation of Factor X. In the cascade of events following vascular injury, F9 is activated by Factor XIa, forming an enzyme complex that catalyzes the activation of Factor X. This activation marks a critical juncture, steering the cascade towards the common pathway shared by both intrinsic and extrinsic pathways. The ultimate outcome is the formation of a stable fibrin clot, arresting bleeding while maintaining blood fluidity under normal circumstances.
F9-Related Diseases
Factor IX deficiency, colloquially known as hemophilia B or Christmas disease, is a consequence of dysregulation or mutations in the F9 gene. Individuals with hemophilia B experience reduced or absent Factor IX activity, leading to a propensity for prolonged bleeding after injuries or surgeries. The severity of hemophilia B varies, with its impact ranging from mild bleeding tendencies to severe, spontaneous bleeding episodes.
Advances in medical science have paved the way for effective management of hemophilia B. Recombinant Factor IX, produced through genetic engineering, has become a cornerstone in treatment, providing a safer alternative to traditional blood-derived clotting factors. The use of replacement therapies has significantly improved the quality of life for individuals with hemophilia B.
F9 Related Signaling Pathways
The signal pathway associated with F9 unfolds in the intricate web of blood coagulation. Triggered by tissue factor exposure due to injury, the intrinsic pathway commences with the activation of Factor XII. F9, activated by Factor XIa in the presence of calcium ions, forms an enzyme complex that activates Factor X. This convergence of pathways culminates in the formation of a stable fibrin clot, preventing uncontrolled bleeding.
Understanding the F9-related signal pathway is essential for deciphering the molecular events governing blood coagulation. This knowledge not only provides insights into the pathophysiology of hemophilia B but also serves as a foundation for developing targeted therapeutic interventions.
Applications of F9 in Biomedical Research
Beyond its role in blood coagulation, F9 has emerged as a promising avenue in biomedical research, with diverse applications on the horizon.
- Recombinant F9 and Gene Therapy
In the realm of hemophilia treatment, recombinant F9 has revolutionized therapeutic strategies. Engineered through biotechnological processes, recombinant F9 offers a safer and more reliable alternative to blood-derived clotting factors. This innovation has significantly improved the accessibility and efficacy of hemophilia B treatment.
Gene therapy, a frontier in biomedical research, holds great promise for individuals with hemophilia B. Researchers are exploring strategies to introduce a functional F9 gene into patients, addressing the underlying genetic defect causing Factor IX deficiency. This approach aims for a sustainable solution, enabling the production of endogenous F9 and potentially alleviating the need for frequent infusions.
- F9 as a Biomarker and Targeted Drug Delivery
The unique properties of the F9 protein also extend its utility beyond blood coagulation. Some studies propose F9 as a potential biomarker for conditions related to blood coagulation. Monitoring changes in F9 levels could offer early indications of disorders, facilitating timely diagnosis and intervention.
Moreover, researchers are investigating the use of F9 for targeted drug delivery. Leveraging the protein's involvement in blood clotting, therapeutic agents can be attached to F9, enabling precise delivery to specific sites in the body. This approach holds promise for minimizing side effects and enhancing the effectiveness of treatments.
The F9 protein, with its intricate structure and multifaceted functions, epitomizes the marvels of molecular biology. From its indispensable role in blood coagulation to its applications in cutting-edge biomedical research, F9 continues to captivate scientists, offering avenues for innovative therapeutic interventions and diagnostic strategies. As research progresses, the journey into the depths of F9 promises to unravel new possibilities for improving human health and well-being.
Recommended Products for F9 Protein
| Cat.# | Species | Product name | Source (Host) | Tag |
|---|---|---|---|---|
| F9-258H | Human | Active Recombinant Human F9 protein, Fc-tagged | HEK293 | Fc |
| F9-4350H | Human | Active Recombinant Human Factor-IX | Human | N/A |
| F9-1157H | Human | Active Recombinant Human F9 protein, His-tagged | HEK293 | His |
| F9-12627H | Human | Recombinant Human F9, His-tagged | E.coli | His |
| F9-3626H | Human | Recombinant Human F9 Protein, GST-tagged | Wheat Germ | GST |
| F9-259H | Human | Recombinant Human F9 protein (Met1-Thr467), hFc-tagged | HEK293 | hFc |
| F9-7808M | Mouse | Recombinant Mouse F9 protein, His-tagged | E.coli | His |
| F9-2181R | Rat | Recombinant Rat F9 Protein | Mammalian Cell | His |
| F9-7809R | Rabbit | Recombinant Rabbit F9 protein, His & GST-tagged | E.coli | His/GST |
| F9-1533R | Rhesus Macaque | Recombinant Rhesus monkey F9 Protein, His-tagged | Mammalian Cell | His |
Reference
- Wang, QY., et al. A genetic analysis of 23 Chinese patients with hemophilia B. Sci Rep. 2016, 6: 25024.

