SpyDirect-a novel biofunctionalization method for enhancing the stability and longevity of electronic biosensors
Electronic biosensors are critical for rapid diagnostics, yet their widespread adoption is hindered by instability in bioreceptor immobilization. Conventional methods, such as SAMs, suffer from oxidative degradation and random orientation of antibodies, compromising sensor performance. The SpyDirect method, presented in this study, addresses these challenges through an innovative biofunctionalization strategy that bypasses SAMs entirely.
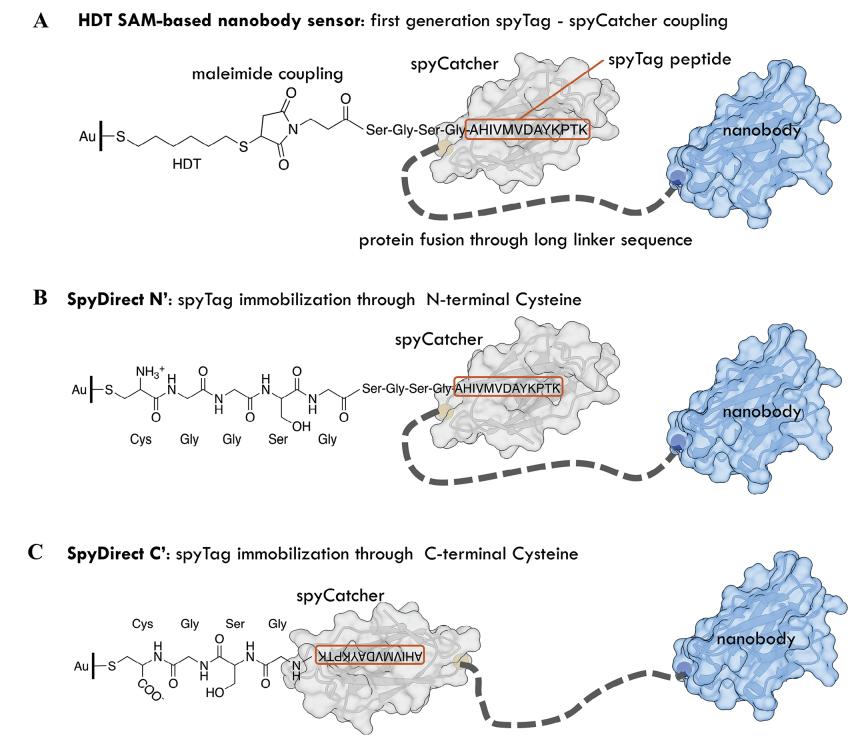
Traditional methods rely on self-assembled monolayers (SAMs) of thiolated molecules to immobilize antibodies on gold electrodes, but these layers degrade rapidly in biological environments, limiting sensor durability. SpyDirect eliminates SAMs by using cysteine-terminated spyTag peptides to directly anchor orientation-optimized nanobodies (small, stable antibody fragments) to gold surfaces. This approach leverages the covalent interaction between spyTag peptides and nanobody-spyCatcher fusion proteins, creating a dense, uniform biorecognition layer. Experimental validation using quartz crystal microbalance (QCM-D), atomic force microscopy (AFM), and electrochemical techniques demonstrated that SpyDirect achieves higher nanobody density, smoother surfaces, reduced nonspecific binding, and superior stability compared to SAM-based methods. Sensors functionalized with SpyDirect detected SARS-CoV-2 spike proteins in complex media like saliva and wastewater with high sensitivity and retained functionality longer during storage. The method's simplicity, eco-friendliness, and scalability highlight its potential for advancing biosensor applications in diagnostics and environmental monitoring.
At the core of SpyDirect is the spyTag/spyCatcher system—a peptide-protein pair that forms irreversible covalent bonds. By engineering spyTag peptides with terminal cysteine residues, the authors directly immobilized these peptides on gold electrodes. Subsequent attachment of nanobody-spyCatcher fusion proteins produced a densely packed, uniformly oriented biorecognition layer. Structural optimization, including C-terminal cysteine placement, minimized steric hindrance, enhancing nanobody density and accessibility.
Experimental validation revealed SpyDirect's superiority. QCM-D showed 2–3 times higher peptide and nanobody densities than SAM-based methods, while AFM confirmed smoother, more homogeneous surfaces. Electrochemical analyses demonstrated reduced background noise and enhanced stability, with SpyDirect sensors retaining 67% functionality after seven days, compared to rapid SAM degradation. Crucially, SpyDirect-enabled organic electrochemical transistors (OECTs) detected SARS-CoV-2 spike proteins at attomolar levels in saliva and wastewater, outperforming commercial rapid tests in sensitivity.
The implications are profound. SpyDirect's aqueous, solvent-free protocol aligns with green chemistry principles, while its ability to store pre-functionalized electrodes simplifies logistics. By improving sensor longevity and adaptability to harsh environments, this method could revolutionize point-of-care diagnostics, implantable devices, and environmental monitoring. Future work may explore broader applications, from detecting other pathogens to integrating SpyDirect with flexible electronics, paving the way for robust, scalable biosensing solutions.
Reference
- Guo K, Grünberg R, Ren Y, Chang T, Wustoni S, Strnad O, Koklu A, Díaz-Galicia E, Agudelo JP, Druet V, Castillo TCH, Moser M, Ohayon D, Hama A, Dada A, McCulloch I, Viola I, Arold ST, Inal S. SpyDirect: A Novel Biofunctionalization Method for High Stability and Longevity of Electronic Biosensors. Adv Sci (Weinh). 2024 Jul;11(27):e2306716. doi: 10.1002/advs.202306716. Epub 2023 Dec 31. PMID: 38161228; PMCID: PMC11251562.
Contact us or send an email at for project quotations and more detailed information.
Quick Links
-

Papers’ PMID to Obtain Coupon
Submit Now -

Refer Friends & New Lab Start-up Promotions

