GroEL
-
Official Full Name
chaperonin GroEL -
Overview
This gene encodes a member of the chaperonin family. The encoded mitochondrial protein may function as a signaling;molecule in the innate immune system. This protein is essential for the folding and assembly of newly imported;proteins in the mitochondria. This gene is adjacent to a related family member and the region between the 2 genes;functions as a bidirectional promoter. Several pseudogenes have been associated with this gene. Two transcript;variants encoding the same protein have been identified for this gene. Mutations associated with this gene cause;autosomal recessive spastic paraplegia 13. -
Synonyms
groEL;chaperonin GroEL;60 kDa chaperonin;groEL protein;groL;mopA;Protein Cpn60;60 kDa chaperone family;promotes refolding of misfolded polypeptides especially under stressful conditions;forms two stacked rings of heptamers to form a barrel-shaped 14mer;ends can be capped by GroES;misfolded proteins enter the barrel where they ar
Recombinant Proteins
- Bacillus subtilis
- S.dysenteriae
- Mycobacterium Tuberculosis
- Staphylococcus
- Streptomyces coelicolor A3(2)
- E.coli
- Francisella tularensis subsp. holarctica (strain LVS)
- E.Coli/Yeast
- E.coli
- His
- Myc
- SUMO
- Non
Background
What is GROEL protein?
GROEL (chaperonin large subunit) gene is a protein coding gene which comes from Bacillus subtilis or other bacteria. This gene is adjacent to a related family member and the region between the 2 genes, functions as a bidirectional promoter. This gene encodes a member of the chaperonin family. The encoded mitochondrial protein may function as a signaling molecule in the innate immune system. The GROEL protein is consisted of 544 amino acids and its molecular mass is approximately 57.4 kDa.
What is the function of GROEL protein?
GROEL protein is a molecular chaperone protein found in many bacteria and eukaryotes. Its main function is to help protein folding, assembly and stability. Specifically, the GroEL protein can bind to these proteins within the cell and promote their proper folding by preventing non-specific aggregation and misfolding. After the protein is folded, the GroEL protein can participate in the assembly process of the protein along with other molecules. The GroEL protein protects the protein from degradation, thereby increasing its stability. Under stressful conditions, expression of the GroEL protein is increased to help cells cope with these adverse environments.
GROEL Related Signaling Pathway
GroEL is a heat shock protein in E. coli and other bacteria that works with another heat shock protein, GroES (chaperonin small subunit), to help the protein assemble and fold. GroEL may also interact with other signaling pathways, for example in relation to processes such as cell cycle regulation, apoptosis, and autophagy.
GROEL Related Diseases
No disease directly related to GroEL protein has been found. However, due to its important role in intracellular protein quality control, some diseases related to protein folding and stability may be related to functional abnormalities of GroEL proteins.
Bioapplications of GROEL
In the field of biotechnology, GroEL can be used as part of protein engineering and expression systems. It can help maintain the stability and correct folding of the target protein, thereby increasing the yield and purity of the recombinant protein. In the field of medical research, the application of GroEL protein is mainly focused on promoting the correct folding of proteins and preventing protein aggregation. This could be a promising direction for the treatment of some neurodegenerative diseases, such as Alzheimer's and Parkinson's
Case Study
Case study 1: Ayako Endo, 2006
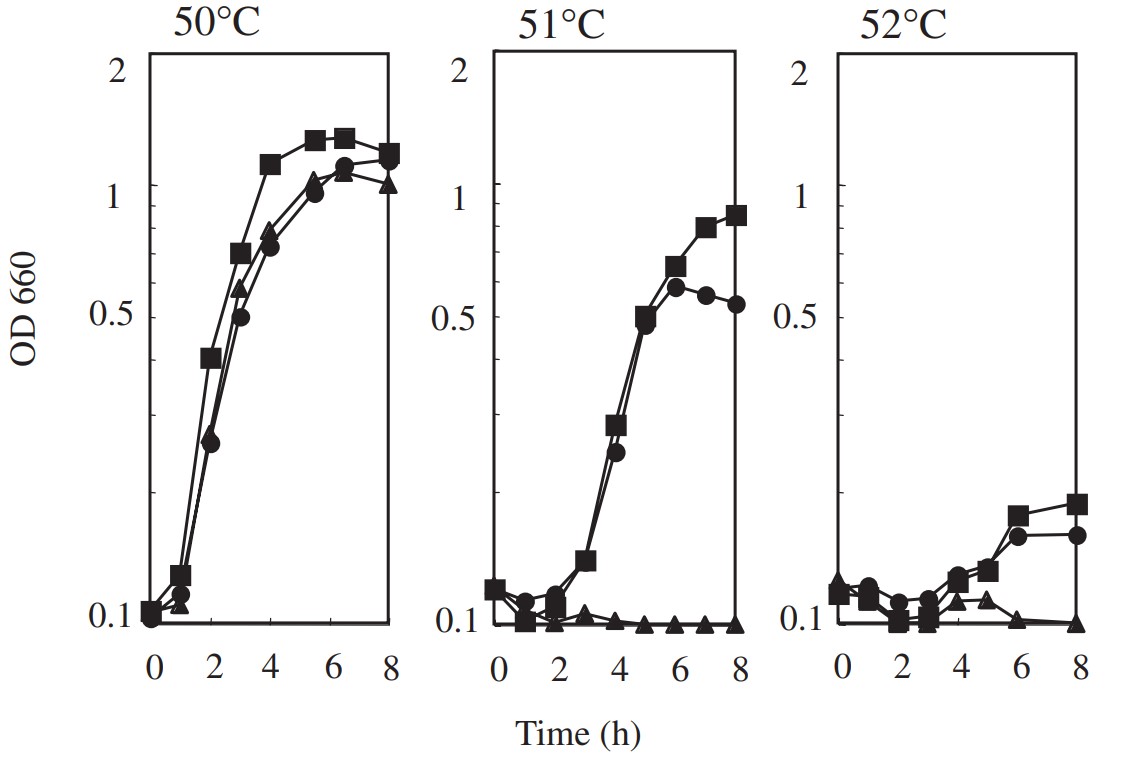
The researchers investigated a temperature adaptation of Bacillus subtilis 168 in which chromosomal groEL was replaced with a psychrophilic groEL. This strain can grow at 50 degrees C but not at 51 degrees C, a temperature at which wild-type B. subtilis can grow. Using in vivo random mutagenesis by the B. subtilis mutator strain (mutS, mutM, mutY), two thermo-adaptants were isolated from the groEL substituted strain at 52 degrees C. They contained novel amino acid alterations in their ATP binding motif (T93I) and the inter-monomer contact (R285H) region of GroEL. These results suggest that GroEL participates in bacterial temperature adaptation.
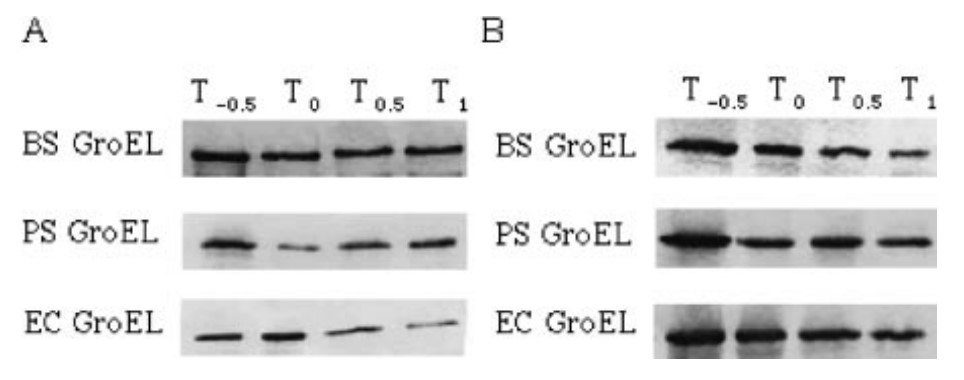
Fig1. Western blot analysis of groEL expression at 37 °C (A) and 50 °C (B).
Case study 2: F Edenhofer, 1996
Prions mediate the pathogenesis of certain neurodegenerative diseases, including bovine spongiform encephalopathy in cattle and Creutzfeldt-Jakob disease in humans. The prion particle consists mainly, if not entirely, of PrPSc, a posttranslationally modified isoform of the cellular host-encoded prion protein (PrPc). Here the researchers employ a Saccharomyces cerevisiae two-hybrid screen to search for proteins which interact specifically with the Syrian golden hamster prion protein. The specificity of the interaction was confirmed in vitro for the recombinant proteins PrPc23-231 and rPrP27-30 fused to glutathione S-transferase with recombinant human Hsp60 as well as the bacterial GroEL. The interaction site for recombinant Hsp60 and GroEL proteins was mapped between amino acids 180 and 210 of the prion protein by screening with a set of recombinant PrPc fragments. The binding of Hsp60 and GroEL occurs within a region which contains parts of the putative alpha-helical domains H3 and H4 of the prion protein.
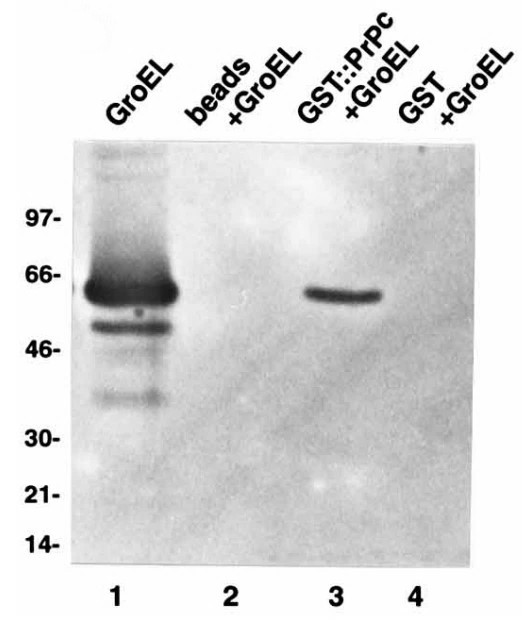
Fig3. Recombinant GST (1 μg) and GST-PrPc (2 μg) immobilized on glutathione-Sepharose as well as glutathione-Sepharose alone were incubated with 25 mg GroEL.
Quality Guarantee
High Purity
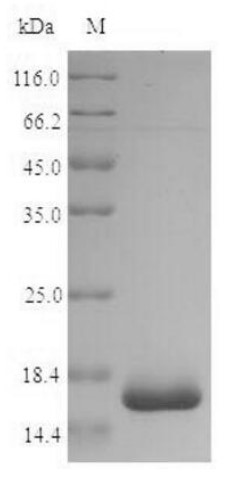
Fig1. SDS-PAGE (groEL-1233S) (PROTOCOL for western blot)
Involved Pathway
GroEL involved in several pathways and played different roles in them. We selected most pathways GroEL participated on our site, such as , which may be useful for your reference. Also, other proteins which involved in the same pathway with GroEL were listed below. Creative BioMart supplied nearly all the proteins listed, you can search them on our site.
| Pathway Name | Pathway Related Protein |
|---|
Protein Function
GroEL has several biochemical functions, for example, . Some of the functions are cooperated with other proteins, some of the functions could acted by GroEL itself. We selected most functions GroEL had, and list some proteins which have the same functions with GroEL. You can find most of the proteins on our site.
| Function | Related Protein |
|---|
Interacting Protein
GroEL has direct interactions with proteins and molecules. Those interactions were detected by several methods such as yeast two hybrid, co-IP, pull-down and so on. We selected proteins and molecules interacted with GroEL here. Most of them are supplied by our site. Hope this information will be useful for your research of GroEL.
Resources
Related Services
Related Products
References
- Arai, T; Ochiai, K; et al. Actinomyces naeslundii GroEL-dependent initial attachment and biofilm formation in a flow cell system. JOURNAL OF MICROBIOLOGICAL METHODS 109:160-166(2015).
- Kaur, G; Chitradevi, C; et al. rIL-22 as an adjuvant enhances the immunogenicity of rGroEL in mice and its protective efficacy against S. Typhi and S. Typhimurium. CELLULAR & MOLECULAR IMMUNOLOGY 12:-(2015).



